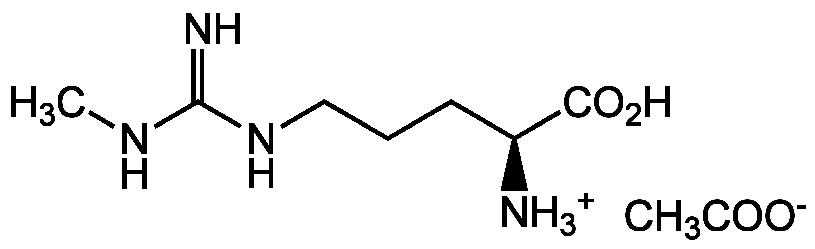
L-NMMA . monoacetate
| Code | Size | Price |
|---|
| AG-CR1-3578-M005 | 5 mg | £30.00 |
Quantity:
| AG-CR1-3578-M025 | 25 mg | £65.00 |
Quantity:
| AG-CR1-3578-M100 | 100 mg | £180.00 |
Quantity:
| AG-CR1-3578-G001 | 1 g | £540.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NG-Monomethyl-L-arginine . monoacetate
Appearance:
White to off-white solid.
CAS:
53308-83-1
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1/C7H16N4O2.C2H4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13;1-2(3)4/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11);1H3,(H,3,4)/t5-;/m0./s1
InChiKey:
IKPNWIGTWUZCKM-JEDNCBNOBD
Long Description:
Chemical. CAS: 53308-83-1. Formula: C7H16N4O2 . CH3COOH. MW: 188.2 . 60.1. Endothelium-derived relaxing factor inhibitor. Inhibits the generation of NO from arginine. Competitive, irreversible non-selective inhibitor of all three NOS I, II, and III isoforms (eNOS, iNOS and nNOS). Inhibits the synthesis of nitric oxide (NO) in a dose-dependent and enantiospecific fashion. Useful tool to study the role and the effects of NO in cardiovascular and gastrointestinal disorders, hypertension, diabetes, septic shock, inflammation, infection, stroke and neurodegenerative disorders.
MDL:
MFCD00069311
Molecular Formula:
C7H16N4O2 . CH3COOH
Molecular Weight:
188.2 . 60.1
Package Type:
Vial
Product Description:
Endothelium-derived relaxing factor inhibitor. Inhibits the generation of NO from arginine. Competitive, irreversible non-selective inhibitor of all three NOS I, II, and III isoforms (eNOS, iNOS and nNOS). Inhibits the synthesis of nitric oxide (NO) in a dose-dependent and enantiospecific fashion. Useful tool to study the role and the effects of NO in cardiovascular and gastrointestinal disorders, hypertension, diabetes, septic shock, inflammation, infection, stroke and neurodegenerative disorders.
Purity:
>99% (TLC)
SMILES:
CC([O-])=O.CNC(=N)NCCC[C@H]([NH3+])C(O)=O
Solubility Chemicals:
Soluble in water, ethanol, methanol or DMSO.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Identification of arginine as a precursor of endothelium-derived relaxing factor: I. Sakuma, et al.; PNAS 85, 8664 (1988) | NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo?: K. Aisaka, et al.; BBRC 160, 881 (1989) | A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation: D.D. Rees, et al.; Br. J. Pharmacol. 96, 418 (1989) | Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo: D.D. Rees, et al.; Br. J. Pharmacol. 101, 746 (1990) | Role of endothelium-formed nitric oxide on vascular responses: J. Marin J & C.F. Sanchez-Ferrer; Gen. Pharmacol. 21, 575 (1990) | Inactivation of macrophage nitric oxide synthase activity by N(G)-methyl-L-arginine: N.M. Olken, et al.; BBRC 177, 828 (1991) | Nitric oxide: an endogenous modulator of leukocyte adhesion: P. Kubes, et al.; PNAS 88, 4651 (1991) | Macrophage nitric oxide mediates immunosuppression in infectious inflammation: T.K. Eisenstein, et al.; Immunobiology 191, 493 (1994) (Review) | N-nitro-L-arginine and N-monomethyl-L-arginine exhibit a differential pattern of inactivation toward the three nitric oxide synthases: D.W. Reif & C.A. McCreedy; Arch. Biochem. Biophys. 320, 170 (1995) | Cell death, survival and proliferation in Tetrahymena thermophila. Effects of insulin, sodium nitroprusside, 8-Bromo cyclic GMP, NG-methyl-L-arginine and methylene blue: S.T. Christensen; Cell Biol. Int. 20, 653 (1996) (Review) | Nitric oxide synthase inhibitors: Amino acids. O.W. Griffith & R.G. Kilbourn; Methods Enzymol. 268, 375 (1996) | Selective inhibitors of neuronal nitric oxide synthase-is no NOS really good NOS for the nervous system? P.K. Moore & R.L. Handy; TIPS 18, 204 (1997) (Review) | Asymmetric dimethylarginine (ADMA) and other endogenous nitric oxide synthase (NOS) inhibitors as an important cause of vascular insulin resistance: K. Toutouzas; Horm. Metab. Res. 40, 655 (2008) (Review) | Insulin resistance after hypertension induced by the nitric oxide synthesis inhibitor L-NMMA in rats: A.D. Baron; Am. J. Physiol. 269, E709 (1995) | The NO synthase inhibitors L-Name and L-NMMA, but not L-arginine, block the mammalian nicotinic acetylcholine receptor channel: M Scheller; Toxicol. Lett. 100-101, 109 (1998) | Induction of apoptosis by L-NMMA, via FKHRL1/ROCK pathway in human gastric cancer cells: Y.Z. Wang & Z.Q. Feng; Biomed. Environ. Sci. 19, 285 (2006)