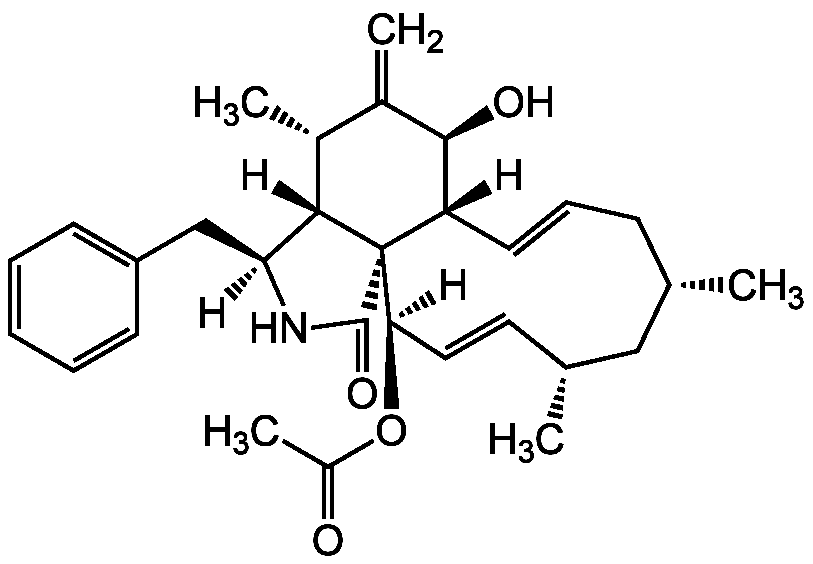
Antibiotic L-696474
| Code | Size | Price |
|---|
| BVT-0331-C500 | 500 ug | £150.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
18-Dehydroxycytochalasin H
Appearance:
White powder.
CAS:
141994-72-1
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Hazards:
H302, H312, H319
InChi:
InChI=1S/C30H39NO4/c1-18-10-9-13-24-28(33)21(4)20(3)27-25(17-23-11-7-6-8-12-23)31-29(34)30(24,27)26(35-22(5)32)15-14-19(2)16-18/h6-9,11-15,18-20,24-28,33H,4,10,16-17H2,1-3,5H3,(H,31,34)/b13-9+,15-14+/t18-,19+,20+,24-,25-,26+,27-,28+,30+/m0/s1
InChiKey:
JVHIPYJQMFNCEK-ZPSJVCBQSA-N
Long Description:
Chemical. CAS: 141994-72-1. Formula: C30H39NO4. MW: 477.6. Isolated from Hypoxylon fragiforme. Natural cytochalasin derivative. Antiviral. Inhibitor of HIV-1 protease. Antifungal activity.
MDL:
MFCD00911041
Molecular Formula:
C30H39NO4
Molecular Weight:
477.6
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Natural cytochalasin derivative. Antiviral. Inhibitor of HIV-1 protease. Antifungal activity.
Purity:
>98% (HPLC; NMR)
Signal Word:
Warning
SMILES:
[H][C@@]1(CC2=CC=CC=C2)NC(=O)[C@]23[C@@]1([H])[C@H](C)C(=C)[C@@H](O)[C@]2([H])C=CC[C@H](C)C[C@H](C)C=CC3([H])OC(C)=O
Solubility Chemicals:
Soluble in DMSO methanol or dichlormethane.
Source / Host:
Isolated from Hypoxylon fragiforme.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
L-696,474, a novel cytochalasin as an inhibitor of HIV-1 protease: I. The producing organism and its fermentation: A.W. Dombrowski, et al.; J. Antibiot. 45, 671 (1992) | L-696,474, a novel cytochalasin as an inhibitor of HIV-1 protease: II. Isolation and structure: J. Ondeyka, et al.; J. Antibiot. 45, 679 (1992) | L-696,474, a novel cytochalasin as an inhibitor of HIV-1 protease: III. Biological activity: R.B. Lingham, et al.; J. Antibiot. 45, 686 (1992) | Microbial transformation of L-696,474, a novel cytochalasin as an inhibitor of HIV-1 protease: T.S. Chen, et al.; J. Nat. Prod. 56, 755 (1993) | An enantioselective, modular, and general route to the cytochalasins: Synthesis of L-696,474 and cytochalasin B: A.M. Haidle & A.G. Myers; PNAS 101, 12048 (2004) | Fungicidal activity of L-696,474 and cytochalasin D from the ascomycete Daldinia concentria against plant pathogenic fungi: L. Du-Qiang, et al.; Acta Phytophyl. Sinica 34, 113 (2007)