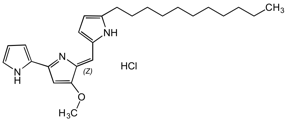
Undecylprodigiosin
| Code | Size | Price |
|---|
| BVT-0422-C250 | 250 ug | £100.00 |
Quantity:
| BVT-0422-M001 | 1 mg | £285.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Undecylprodiginine; Prodigiosin 25C
Appearance:
Red solid.
CAS:
56247-02-0 | 52340-48-4 (parent)
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light.
InChi:
InChI=1S/C25H35N3O.ClH/c1-3-4-5-6-7-8-9-10-11-13-20-15-16-21(27-20)18-24-25(29-2)19-23(28-24)22-14-12-17-26-22;/h12,14-19,26-27H,3-11,13H2,1-2H3;1H/b24-18-;
InChiKey:
ZZRPZWXEPLULCE-XWASNXTISA-N
Long Description:
Chemical. CAS: 56247-02-0 | 52340-48-4 (parent). Formula: C25H35N3O . HCl. MW: 393.6 . 36.5. Isolated from Streptomyces parvulus. Tripyrrolic pigment like prodigiosin. Antimalarial agent. Apoptosis inducer. Anticancer agent with multiple modes of action. Immunosuppressant in non-toxic concentrations. Bone resorption inhibitor. Antiulcer agent.
MDL:
MFCD30738199
Molecular Formula:
C25H35N3O . HCl
Molecular Weight:
393.6 . 36.5
Package Type:
Plastic Vial
Product Description:
Tripyrrolic pigment like prodigiosin. Antimalarial agent. Apoptosis inducer. Anticancer agent with multiple modes of action. Immunosuppressant in non-toxic concentrations. Bone resorption inhibitor. Antiulcer agent.
Purity:
>95% (HPLC, NMR)
SMILES:
COC1=CC(C2=CC=CN2)=N/C1=CC3=CC=C(CCCCCCCCCCC)N3.Cl
Solubility Chemicals:
Soluble in DMSO, methanol, acetone or chloroform.
Source / Host:
Isolated from Streptomyces parvulus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
A new prodiginine (prodigiosin-like) pigment from Streptomyces. Antimalarial activity of several prodiginines: N. N. Gerber; J. Antibiot. 28, 194 (1975) | Undecylprodigiosin: H. H. Wassermann, et al.; Tetrahedron 32, 1851 (1976) | Characterization of the new immunosuppressive drug undecylprodigiosin in human lymphocytes: retinoblastoma protein, cyclin-dependent kinase-2, and cyclin-dependent kinase-4 as molecular targets: S. Songia, et al.; J. Immunol. 158, 3987 (1997) | Chemistry and biology of roseophilin and the prodigiosin alkaloids: a survey of the last 2500 years: A. F?rstner; Angew. Chem. Int. Ed. 42, 3582 (2003) | The prodigiosins: a new family of anticancer drugs: B. Montaner & R. P?rez-Tomas; Curr. Cancer Drug Targets 3, 57 (2003) (Review) | Potent in vitro anticancer activity of metacycloprodigiosin and undecylprodigiosin from a sponge-derived actinomycete Saccharopolyspora sp. nov: R. Liu, et al.; Arch. Pharm. Res. 28, 1341 (2005) | The biosynthesis and regulation of bacterial prodiginines: N. R. Williamson, et al.; Nat. Rev. Microbiol. 4, 887 (2006) (Review) | Undecylprodigiosin selectively induces apoptosis in human breast carcinoma cells independent of p53: T.-F. Ho, et al.; Toxicol. Appl. Pharmacol. 225, 318 (2007) | Prodigiosin synthesis with electron rich 2,2`-bipyrroles: B. Jolicoeur & W. D. Lubell; Can. J. Chem. 86, 213 (2008) | Antimalarial activity of natural and synthetic prodiginines: K. Papireddy, et al.; J. Med. Chem. 54, 5296 (2011) | Streptomyces sp. JS520 produces exceptionally high quantities of undecylprodigiosin with antibacterial, antioxidative, and UV-protective properties: N. Stankovic, et al.; Appl. Microbiol. Biotechnol. 96, 1217 (2012) | Undecylprodigiosin induced apoptosis in P388 cancer cells is associated with its binding to ribosome: P. Liu, et al.; PLoS One 8, e65381 (2013) | Properties and applications of undecylprodigiosin and other bacterial prodigiosins: N. Stankovic, et al.; Appl. Microbiol. Biot. 98, 3841 (2014) | Undecylprodigiosin conjugated monodisperse gold nanoparticles efficiently cause apoptosis in colon cancer cells in vitro: J. Nikodinovic-Runic, et al.; J. Mater. Chem. B: Mat. Biol. Med. 2, 3271 (2014) | Stereochemistry and Mechanism of undecylprodigiosin Oxidative Carbocyclization to Streptorubin B by the rieske oxygenase RedG: D.M. Withall, et al.; JACS 137, 7889 (2015) | The Streptomyces metabolite anhydroexfoliamycin ameliorates hallmarks of Alzheimer's disease in vitro and in vivo: M. Leiros, et al.; Neuroscience (Amsterdam, Netherlands) 305, 26 (2015) | Biological effects of bacterial pigment undecylprodigiosin on human blood cells treated with atmospheric gas plasma in vitro: S. Lazovic, et al.; Exp. Toxicol. Pathol. 69, 55 (2017)