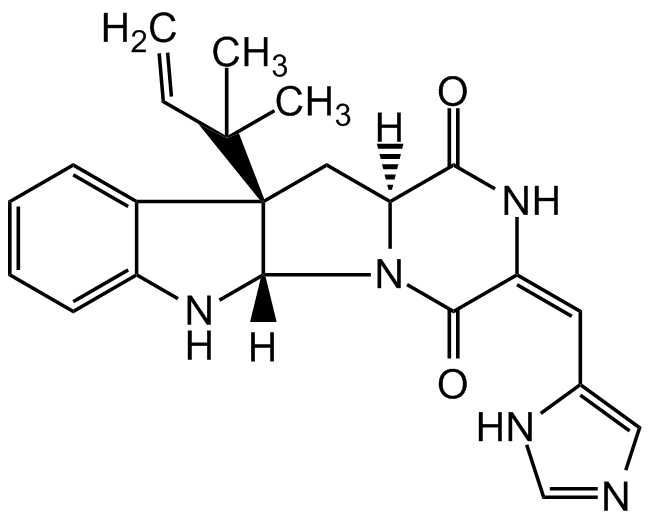
Roquefortine C
| Code | Size | Price |
|---|
| BVT-0425-C500 | 500 ug | £110.00 |
Quantity:
| BVT-0425-M001 | 1 mg | £190.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NSC292134
Appearance:
White to off-white solid.
CAS:
58735-64-1
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Protect from light.
Hazards:
H301, H311, H331
InChi:
InChI=1S/C22H23N5O2/c1-4-21(2,3)22-10-17-18(28)25-16(9-13-11-23-12-24-13)19(29)27(17)20(22)26-15-8-6-5-7-14(15)22/h4-9,11-12,17,20,26H,1,10H2,2-3H3,(H,23,24)(H,25,28)/b16-9+/t17-,20-,22+/m0/s1
InChiKey:
SPWSUFUPTSJWNG-JJUKSXGLSA-N
Long Description:
Chemical. CAS: 58735-64-1. Formula: C22H23N5O2. MW: 389.5. Isolated from Penicillium sp. Potent neurotoxin. Mycotoxin. Tremorgenic. Gram-positive bacteria growth inhibitor. Cytochrome p450 inhibitor. Lymphocyte proliferation inhibitor. Cytotoxic.
MDL:
MFCD22416530
Molecular Formula:
C22H23N5O2
Molecular Weight:
389.5
Package Type:
Plastic Vial
PG:
III
Precautions:
P280, P301, P312, P302, P352, P305, P351, P338
Product Description:
Potent neurotoxin. Mycotoxin. Tremorgenic. Gram-positive bacteria growth inhibitor. Cytochrome p450 inhibitor. Lymphocyte proliferation inhibitor. Cytotoxic.
Purity:
>98% (HPLC, NMR)
Signal word:
Danger
SMILES:
[H][C@@]12C[C@]3(C4=CC=CC=C4N[C@@]3([H])N1C(=O)C(NC2=O)=C/C1=CN=CN1)C(C)(C)C=C
Solubility Chemicals:
Soluble in DMSO, methanol, 100% ethanol, dimethylformamide, acetone, chloroform or dichloromethane.
Source / Host:
Isolated from Penicillium sp.
Transportation:
Excepted Quantity
UN Nummer:
UN 3172
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
Isolation of festuclavine and three new indole alkaloids, roquefortine A, B and C from the cultures of Penicillium roqueforti: S. Ohmomo, et al.; Agric. Biol. Chem. 39, 1333 (1975) | Roquefortine and isofumigaclavine A, metabolites from Penicillium roqueforti: P.M. Scott, et al.; Experientia 32, 140 (1976) | Identification of roquefortine C produced by Penicillium roqueforti: S. Ohmomo et al.; Agric. Biol. Chem. 41, 2097 (1977) | Antimicrobial action of roquefortine: B. Kopp-Holtwiesche & H.J. Rehm; J. Environ. Pathol. Toxicol. Oncol. 10, 41 (1990) | MS/MS screen for the tremorgenic mycotoxins roquefortine and penitrem A: W.E. Braselton & P.C. Rumler; J. Vet. Diagn. Invest. 8, 515 (1996) | Molecular requirements for inhibition of cytochrome p450 activities by roquefortine: C. Aninat, et al.; Chem. Res. Toxicol. 14, 1259 (2001) | The effects of the Penicillium mycotoxins citrinin, cyclopiazonic acid, ochratoxin A, patulin, penicillic acid, and roquefortine C on in vitro proliferation of porcine lymphocytes: M. Keblys, et al.; Mycopathologia 158, 317 (2004) | Combined effects of selected Penicillium mycotoxins on in vitro proliferation of porcine lymphocytes: A. Bernhoft, et al.; Mycopathologia 158, 441 (2004) | Cytotoxicity of occupationally and environmentally relevant mycotoxins: J. Bunger, et al.; Toxicology 202, 199 (2004) | Application of Ambient Ionization Mass Spectrometry to Detect the Mycotoxin Roquefortine C in Blue Cheese: C.M. Maragos; Food Analyt. Meth. 45, (2021)
Related Products
| Product Name | Product Code | Supplier | Rubrofusarin | BVT-0395 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Helvolic acid | BVT-0435 | Bioviotica | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||