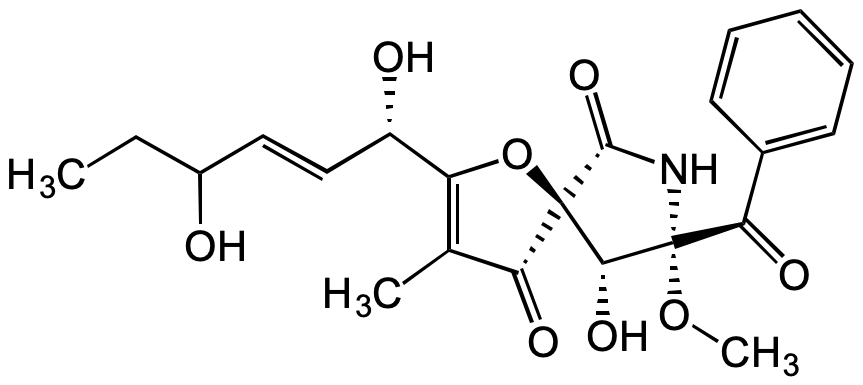
Pseurotin D
| Code | Size | Price |
|---|
| BVT-0426-M001 | 1 mg | £140.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Appearance:
Off-white to beige solid.
CAS:
77409-68-8
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Hazards:
H302, H312, H319
InChi:
InChI=1S/C22H25NO8/c1-4-14(24)10-11-15(25)16-12(2)17(26)21(31-16)19(28)22(30-3,23-20(21)29)18(27)13-8-6-5-7-9-13/h5-11,14-15,19,24-25,28H,4H2,1-3H3,(H,23,29)/b11-10+/t14?,15-,19+,21+,22+/m0/s1
InChiKey:
GZAYJWNDYQINEI-OLSIJDASSA-N
Long Description:
Chemical. CAS: 77409-68-8. Formula: C22H25NO8. MW: 431.4. Isolated from Aspergillus fumigatus. Isomeric to pseurotin A (Prod. No. BVT-0003). Anticancer and antiparasitic compound. Apomorphine antagonist. Neuroleptic agent. Inhibitor of IgE production.
MDL:
N/A
Molecular Formula:
C22H25NO8
Molecular Weight:
431.4
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Isomeric to pseurotin A (Prod. No. BVT-0003). Anticancer and antiparasitic compound. Apomorphine antagonist. Neuroleptic agent. Inhibitor of IgE production. Decreases ovalbumin-induced foot pad edema in vivo. Inhibits activation of B-cells and differentiation into the plasma cells. Apoptosis inducer.
Purity:
>98% (HPLC, NMR)
Signal Word:
Warning
SMILES:
CC1=C([C@@H](O)/C=C/C(O)CC)O[C@@]2([C@@H](O)[C@](OC)(C(C3=CC=CC=C3)=O)NC2=O)C1=O
Solubility Chemicals:
Soluble in DMSO, chloroform or dichloromethane.
Source / Host:
Isolated from Aspergillus fumigatus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
Pseurotin B, C, D and E. Further new metabolites of Pseudeurotium ovalis STOLK: W. Breitenstein et al.; Helv. Chim. Acta 64, 379 (1981) | Biologically active pseurotins A and D, new metabolites from Aspergillus fumigates, process for their preparation and their use as apomorphine antagonist: J. Wink, et al.; Eur. Pat. Appl. EP546475A1 (1993) | Pseurotin A and its analogues as inhibitors of immunoglobulin E production: M. Ishikawa, et al.; Bioorg. Med. Chem. Lett. 19, 1457 (2009) | Antitumor compositions containing pseurotin D: D. G. Kim, et al.; Repub. Korean Kongkae aeho Kongbo KR20110087395 (2011) | Antiparasitic and anticancer constituents of the endophytic fungus Aspergillus sp. strain F1544: S. Martinez-Luis, et al.; Nat. Prod. Comm. 7, 165 (2012) | Elucidation of Pseurotin Biosynthetic Pathway Points to Trans-Acting C-Methyltransferase: Generation of Chemical Diversity: Y. Tsunematsu, et al.; Angew. Chem. Int. Ed. 53, 8475 (2014) | Angiogenesis Inhibitors and Anti-Inflammatory Agents from Phoma sp. NTOU4195: M.-S. Lee, et al.; J. Nat. Prod. 79, 2983 (2016) | Treatment of epilepsy using pseurotins and azaspirofurans: D. Copmans, et al.; PCT Int. Appl. WO 2019043019 A1 20190307 (2019) | Natural pseurotins and analogs thereof inhibit activation of B-cells and differentiation into the plasma cells: O. Vasicek, et al.; Phytomedicine in press (2020) | Pseurotin D Induces Apoptosis through Targeting Redox Sensitive Pathways in Human Lymphoid Leukemia Cells: E. Mosejova, et al.; Antioxidants 10, 1576 (2021)