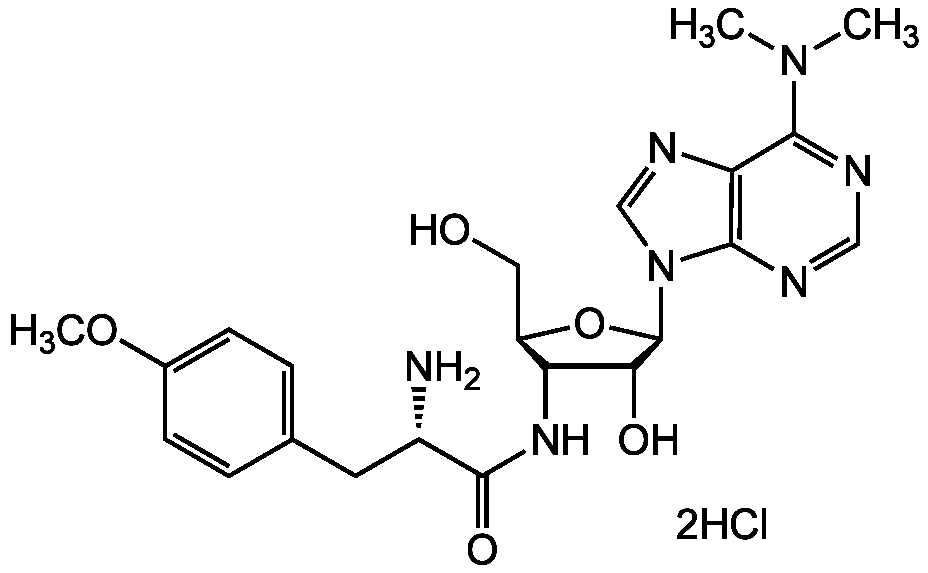
Puromycin . 2HCl
Product Code: AG-CN2-0078
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0078-M025 | 25 mg | £40.00 |
Quantity:
| AG-CN2-0078-M100 | 100 mg | £105.00 |
Quantity:
| AG-CN2-0078-M250 | 250 mg | £180.00 |
Quantity:
| AG-CN2-0078-M500 | 500 mg | £320.00 |
Quantity:
| AG-CN2-0078-G001 | 1 g | £500.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Images
Documents
Further Information
Alternate Names/Synonyms:
CL16,536; NSC 3055; Stylomycin
Appearance:
White to off-white powder.
CAS:
58-58-2
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from moisture.
Hazards:
H302
InChi:
InChI=1/C22H29N7O5/c1-28(2)19-17-20(25-10-24-19)29(11-26-17)22-18(31)16(15(9-30)34-22)27-21(32)14(23)8-12-4-6-13(33-3)7-5-12/h4-7,10-11,14-16,18,22,30-31H,8-9,23H2,1-3H3,(H,27,32)/t14-,15+,16?,18?,22+/m0/s1
InChiKey:
RXWNCPJZOCPEPQ-NIAQAADTBJ
Long Description:
Chemical. CAS: 58-58-2. Formula: C22H29N7O5 . 2HCl. MW: 471.5 . 72.9. Isolated from Streptomyces alboniger. Aminonucleoside antibiotic. Protein synthesis inhibitor. Disrupts peptide transfer on ribosomes (acting as an acyl-tRNA analog) causing premature chain termination during translation. Translational inhibitor in prokaryotic and eukaryotic cells in both in vitro and in vivo systems. Inhibits the transport of proteins into the mitochondria in vitro. Reversible inhibitor of dipeptidyl-peptidase II (serine peptidase) and cytosol alanyl aminopeptidase (metallopeptidase). Apoptosis inducer. Inhibits the growth of Gram-positive bacteria, various animal and insect cells. Fungi and Gram-negative bacteria are resistant due to the low permeability to the antibiotic. Antineoplastic agent. Used in cell biology as selective agent in cell culture systems. It allows selection for cells that contain the resistance gene puromycin N-acetyl-transferase (PAC). Puromycin has a fast mode of action, causing rapid cell death at low antibiotic concentrations. Adherent mammalian cells are sensitive to concentrations of 2 to 5 µg/ml, while cells in suspension are sensitive to concentrations as low as 0.5 to 2 µg/ml. Puromycin-resistant stable mammalian cell lines can be generated in less than one week.
MDL:
MFCD00012691
Molecular Formula:
C22H29N7O5 . 2HCl
Molecular Weight:
471.5 . 72.9
Other data:
NOTE: The working puromycin concentration for selection in mammalian cell lines ranges from 1-10 µg/ml. Prior to using the puromycin antibiotic, titrate the selection agent to determine the optimal concentration for target cell line. Use the lowest concentration that kills 100% of non-transfected cells in 3-5 days from the start of puromycin selection. See also online selection-protocol.
Package Type:
Vial
Precautions:
P264, P301, P312, P330
Product Description:
Aminonucleoside antibiotic. Protein synthesis inhibitor. Disrupts peptide transfer on ribosomes (acting as an acyl-tRNA analog) causing premature chain termination during translation. Translational inhibitor in prokaryotic and eukaryotic cells in both in vitro and in vivo systems. Inhibits the transport of proteins into the mitochondria in vitro. Reversible inhibitor of dipeptidyl-peptidase II (serine peptidase) and cytosol alanyl aminopeptidase (metallopeptidase). Apoptosis inducer. Inhibits the growth of Gram-positive bacteria, various animal and insect cells. Fungi and Gram-negative bacteria are resistant due to the low permeability to the antibiotic. Antineoplastic agent. Used in cell biology as selective agent in cell culture systems. It allows selection for cells that contain the resistance gene puromycin N-acetyl-transferase (PAC). Puromycin has a fast mode of action, causing rapid cell death at low antibiotic concentrations. Adherent mammalian cells are sensitive to concentrations of 2 to 5 µg/ml, while cells in suspension are sensitive to concentrations as low as 0.5 to 2 µg/ml. Puromycin-resistant stable mammalian cell lines can be generated in less than one week.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
COC1=CC=C(C[C@H](N)C(=O)NC2[C@@H](CO)O[C@H]([C@H]2O)N2C=NC3=C2N=CN=C3N(C)C)C=C1
Solubility Chemicals:
Soluble in water.
Source / Host:
Isolated from Streptomyces alboniger.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Puromycin inhibition of protein synthesis: M.A. Darken; Pharmacol. Rev. 16, 223 (1964) (Review) | Mechanism of puromycin action: fate of ribosomes after release of nascent polypeptide chains from polysomes: M.E. Azzam & D. Algranati; PNAS 70, 3866 (1973) | Inhibition of aminopeptidase and acetylcholinesterase by puromycin and puromycin analogs: L.B. Hersh; J. Neurochem. 36, 1594 (1981) | Isolation and properties of a puromycin acetyltransferase from puromycin-producing Streptomyces alboniger: S.Y. Paik, et al.; J. Antibiot. (Tokyo) 38, 1761 (1985) | Biosynthesis of puromycin by Streptomyces alboniger. Characterization of puromycin N-acetyltransferase: J. Vara, et al.; Biochemistry 24, 8074 (1985) | Molecular analysis of the pac gene encoding a puromycin-N-acetyl transferase from Streptomyces alboniger: R.A. Lacalle, et al.; Gene 79, 375 (1989) | Use of puromycin N-acetyltransferase (PAC) as a new reporter gene in transgenic animals: E. Gomez Lahoz, et al.; Nucleic Acids Res. 19, 3465 (1991) | Pac gene as efficient dominant marker and reporter gene in mammalian cells: S. De La Luna & J. Ortin; Meth. Enzymol. 216, 376 (1992) | Puromycin reaction for the A site-bound peptidyl-tRNA: Y. Semenkov, et al.; FEBS Lett. 296, 207 (1992) | Unexpected cytokinetic effects induced by puromycin include a G2-arrest, a metaphase-mitotic-arrest, and apoptosis: A.N. Davidoff & B.V. Mendelow; Leuk. Res. 16, 1077 (1992) | Puromycin is a potent and specific inhibitor of tyrosine kinase activity in HL-60 cells: A.N. Davidoff & B.V. Mendelow; Anticancer Res. 12, 1761 (1992) | Puromycin inhibits protein import into mitochondria by interfering with an intramitochondrial ATP-dependent reaction: J. Price & K. Verner; Biochim. Biophys. Acta 1150, 89 (1993) | Puromycin-sensitive aminopeptidase. Sequence analysis, expression, and functional characterization: D.B. Constam, et al.; J. Biol. Chem. 270, 26931 (1995) | A simple assay for puromycin N-acetyltransferase: selectable marker and reporter: C. Mielke, et al.; Trends Genet. 11, 258 (1995) | Cell proliferation and apoptosis of the glomerular epithelial cells in rats with puromycin aminonucleoside nephrosis: H. Shiiki, et al.; Pathobiology 66, 221 (1998) | Puromycin Aminonucleoside Induces Glomerular Epithelial Cell Apoptosis: V. Sanwal, et al.; Exp. Mol. Pathol. 70, 54 (2001) | Increased Apoptosis in Acute Puromycin Aminonucleoside Nephrosis: L. Fernandez, et al.; Exp. Nephrol. 9, 99 (2001)
Related Products
| Product Name | Product Code | Supplier | G418 . sulfate | AG-CN2-0030 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colistin sulfate (USP Grade) | AG-CN2-0065 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gentamicin sulfate (USP Grade) | AG-CN2-0066 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||