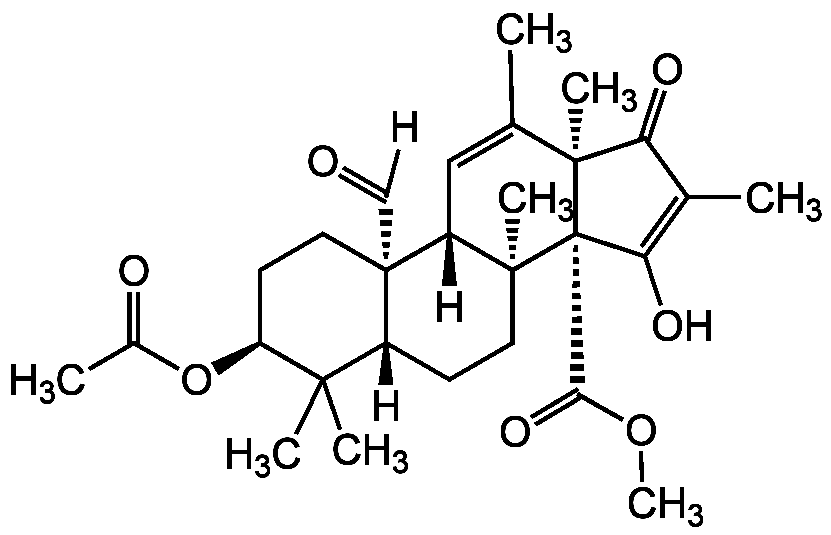
Andrastin A
Product Code: AG-CN2-0144
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0144-C250 | 250 ug | £90.00 |
Quantity:
| AG-CN2-0144-M001 | 1 mg | £250.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Appearance:
Off-white solid.
CAS:
174232-42-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302, H312, H319
InChi:
InChI=1S/C28H38O7/c1-15-13-19-25(6,28(23(33)34-8)22(32)16(2)21(31)26(15,28)7)11-9-18-24(4,5)20(35-17(3)30)10-12-27(18,19)14-29/h13-14,18-20,32H,9-12H2,1-8H3/t18-,19-,20+,25+,26+,27+,28-/m1/s1
InChiKey:
SNSSSENJBPCLPM-OXILWVMOSA-N
Long Description:
Chemical. CAS: 174232-42-9. Formula: C28H38O7. MW: 486.6. Isolated from Aspergillus fumigatus. Mycotoxin. Protein farnesyltransferase (PFTase) inhibitor. Anti-cancer compound. Directly interacts with P-glycoprotein and inhibits the efflux of antitumor agents in drug resistant cells.
MDL:
MFCD28898503
Molecular Formula:
C28H38O7
Molecular Weight:
486.6
Package Type:
Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Mycotoxin [1, 5, 7, 8]. Protein farnesyltransferase (PFTase) inhibitor [1-3]. Anti-cancer compound [1-3]. Directly interacts with P-glycoprotein and inhibits the efflux of antitumor agents in drug resistant cells [4, 6].
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
[H]C(=O)[C@@]12CC[C@H](OC(C)=O)C(C)(C)[C@@]1([H])CC[C@@]1(C)[C@@]2([H])C=C(C)[C@@]2(C)C(=O)C(C)=C(O)[C@@]12C(=O)OC
Solubility Chemicals:
Soluble in DMSO, methanol, ethanol, ethyl acetate or chloroform. Insoluble in water or hexane.
Source / Host:
Isolated from Aspergillus fumigatus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Andrastins A-C, new protein farnesyltransferase inhibitors produced by Penicillium sp. FO-3929. I. Producing strain, fermentation, isolation, and biological activities: S. Omura, et al.; J. Antibiot. 49, 414 (1996) | Andrastins A-C, new protein farnesyltransferase inhibitors produced by Penicillium sp. FO-3929. II. Structure and biosynthesis: R. Uchida, et al.; J. Antibiot. 49, 414 (1996) | Andrastins A-C, New Protein Farnesyltransferase Inhibitors, Produced by Penicillium sp. FO-3929: K. Shiomi, et al.; THL 37, 1265 (1996) | Enhancement of drug accumulation by andrastin A produced by Penicillium sp. FO-3929 in vincristine-resistant KB cells: M.C. Rho, et al.; J. Antibiot. 51, 68 (1998) | Andrastins A-D, Penicillium roqueforti Metabolites consistently produced in blue-mold-ripened cheese: K.F. Nielsen, et al.; J. Agric. Food Chem. 53, 2908 (2005) | Andrastin A and barceloneic acid metabolites, protein farnesyl transferase inhibitors from Penicillium albocoremium: chemotaxonomic significance and pathological implications: D.P. Overy, et al.; Mycol. Res. 109, 1243 (2005) | Proteolytic activity, mycotoxins and andrastin A in Penicillium roqueforti strains isolated from Cabrales, Valdeon and Bejes; Tresviso local varieties of blue-veined cheeses: M.A. Fernandez-Bodega, et al.; Int. J. Food Microbiol. 136, 18 (2009) | In vitro cytotoxicity of fungi spoiling maize silage: R.R. Rasmussen, et al.; Food Chem. Tox. 49, 31 (2011)
Related Products
| Product Name | Product Code | Supplier | Fuscin | AG-CN2-0138 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neoxaline | AG-CN2-0154 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T-2 Toxin | AG-CN2-0473 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||