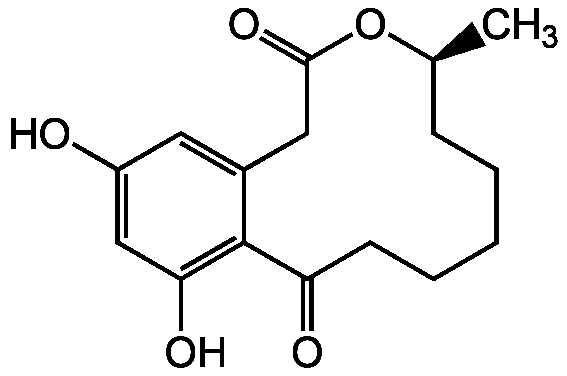
Curvularin
Product Code: AG-CN2-0147
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0147-M001 | 1 mg | £65.00 |
Quantity:
| AG-CN2-0147-M005 | 5 mg | £230.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(S)-Curvularin; NSC 166071
Appearance:
Off-white solid.
CAS:
10140-70-2
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C16H20O5/c1-10-5-3-2-4-6-13(18)16-11(8-15(20)21-10)7-12(17)9-14(16)19/h7,9-10,17,19H,2-6,8H2,1H3/t10-/m0/s1
InChiKey:
VDUIGYAPSXCJFC-JTQLQIEISA-N
Long Description:
Chemical. CAS: 10140-70-2. Formula: C16H20O5. MW: 292.3. Isolated from Penicillium sp. FKI-1938 Antibiotic. Antiprotozoal. Antifungal and phytotoxic compound. Anti-inflammatory. TGF-beta signaling inhibitor Anticancer compound. Inhibits expression of inducible nitric oxide synthase (iNOS; NOSII). Cell division inhibitor. Acetylcholinesterase (AChE) inhibitor.
MDL:
MFCD09752718
Molecular Formula:
C16H20O5
Molecular Weight:
292.3
Package Type:
Vial
Product Description:
Antibiotic [1]. Antiprotozoal [1]. Antifungal and phytotoxic compound [1, 6]. Anti-inflammatory [7]. TGF-beta signaling inhibitor [8] Anticancer compound [2, 9]. Inhibits expression of inducible nitric oxide synthase (iNOS; NOSII) [4, 5]. Cell division inhibitor [3]. Acetylcholinesterase (AChE) inhibitor [9].
Purity:
>95% (HPLC)
SMILES:
C[C@H]1CCCCCC(=O)C2=C(O)C=C(O)C=C2CC(=O)O1
Solubility Chemicals:
Soluble in ethanol, methanol, DMSO, dioxane or pyridine. Insoluble in water, hexane, benzene or chloroform.
Source / Host:
Isolated from Penicillium sp. FKI-1938
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Antimicrobial properties of fungal macrolide antibiotics: V. Betina & D. Micekova; Z. Allg. Mikrobiol. 12, 355 (1972) | Curvularin from Penicillium baradicum Baghdadi NRRL 3754, and biological effects: R.F. Vesonder, et al.; J. Environ. Sci. Health 11, 289 (1976) | Structural study of curvularin, a cell division inhibitor: E.L. Ghisalberti, et al.; Austral. J. Chem. 46, 571 (1993) | Sporogen, S14-95, and S-curvularin, three inhibitors of human inducible nitric-oxide synthase expression isolated from fungi: Y. Yao, et al.; Mol. Pharmacol. 63, 383 (2003) | Inhibitors of inducible NO synthase expression: total synthesis of (S)-curvularin and its ring homologues: S. Elzner, et al.; ChemMedChem 3, 924 (2008) | Isolation and Difference in Anti-Staphylococcus aureus Bioactivity of Curvularin Derivates from Fungus Eupenicillium sp.: L.W. Xie, et al.; Appl. Biochem. Biotechnol. 159, 284 (2009) | The anti-inflammatory fungal compound (S)-curvularin reduces proinflammatory gene expression in an in vivo model of rheumatoid arthritis: N. Schmidt, et al.; J. Pharmacol. Exp. Ther. 343, 106 (2012) | Inhibition of TGF-beta signaling by the fungal lactones (S)-curvularin, dehydrocurvularin, oxacyclododecindione and galiellalactone: K. Rudolph, et al.; Cytokine 61, 285 (2013) | Metabolite profiling and biological activities of bioactive compounds produced by Chrysosporium lobatum strain BK-3 isolated from Kaziranga National Park, Assam, India: C.G. Kumar, et al.; SpringerPlus 2, 122 (2013)
Related Products
| Product Name | Product Code | Supplier | 10,11-Dehydrocurvularin | AG-CN2-0165 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|