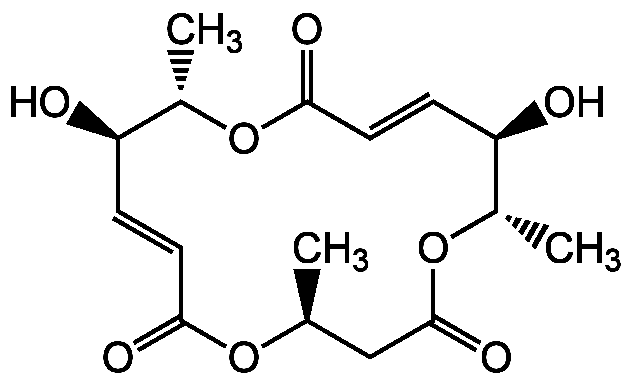
Macrosphelide A
Product Code: AG-CN2-0152
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0152-M001 | 1 mg | £130.00 |
Quantity:
| AG-CN2-0152-M005 | 5 mg | £490.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Appearance:
White solid.
CAS:
172923-77-2
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light.
Hazards:
H302, H312, H319
InChi:
InChI=1S/C16H22O8/c1-9-8-16(21)24-11(3)13(18)5-7-15(20)23-10(2)12(17)4-6-14(19)22-9/h4-7,9-13,17-18H,8H2,1-3H3/b6-4-,7-5-/t9-,10-,11-,12+,13+/m0/s1
InChiKey:
MJMMUATWVTYSFD-MGQLGLDKSA-N
Long Description:
Chemical. CAS: 172923-77-2. Formula: C16H22O8. MW: 342.4. Isolated from Paraconiothyrium sporulosum FO-5050. Antibiotic. Cell-cell adhesion inhibitor. Antimetastatic compound. Antifungal. Antimicrobial.
MDL:
MFCD28898502
Molecular Formula:
C16H22O8
Molecular Weight:
342.4
Package Type:
Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Antibiotic [1, 2]. Cell-cell adhesion inhibitor [1, 2]. Antimetastatic compound [1, 2, 5]. Antifungal [3]. Antimicrobial [4].
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
C[C@H]1CC(=O)O[C@@H](C)[C@H](O)C=CC(=O)O[C@@H](C)[C@H](O)C=CC(=O)O1
Solubility Chemicals:
Soluble in methanol, DMSO, chloroform, ethyl acetate or dimethyl ether. Insoluble in water or hexane.
Source / Host:
Isolated from Paraconiothyrium sporulosum FO-5050.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Macrosphelide, a novel inhibitor of cell-cell adhesion molecule. I. Taxonomy, fermentation, isolation and biological activities: M. Hayashi, et al.; J. Antibiot. 48, 1435 (1995) | Macrosphelide, a novel inhibitor of cell-cell adhesion molecule. II. Physiochemical properties and structural elucidation: S. Takamatsu, et al.; J. Antibiot. 49, 95 (1996) | Production of macrosphelide A by the mycoparasite Coniothyrium minitans: MP. McQuilken, et al.; FEMS Microbiol. Lett. 14, 219 (2003) | Antimicrobial activity of Coniothyrium minitans and its macrolide antibiotic macrosphelide A: N. Tomprefa, et al.; J. Appl. Microbiol. 106, 2048 (2009) | Enantioselective total synthesis of macrosphelides A and E: K.R. Prasad & P. Gutala; Tetrahedron 67, 4514 (2011) | Development of an advanced synthetic route to macrosphelides and its application to the discovery of a more potent macrosphelide derivative: Y.M. Heo, et al.; Molecules 19, 15572 (2014) | Design and synthesis of a macrosphelide A-biotin chimera: H. Yun, et al.; Org. Biomol. Chem. 12, 7127 (2014)
Related Products
| Product Name | Product Code | Supplier | Nocardamine | AG-CN2-0150 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NG 012 | AG-CN2-0155 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||