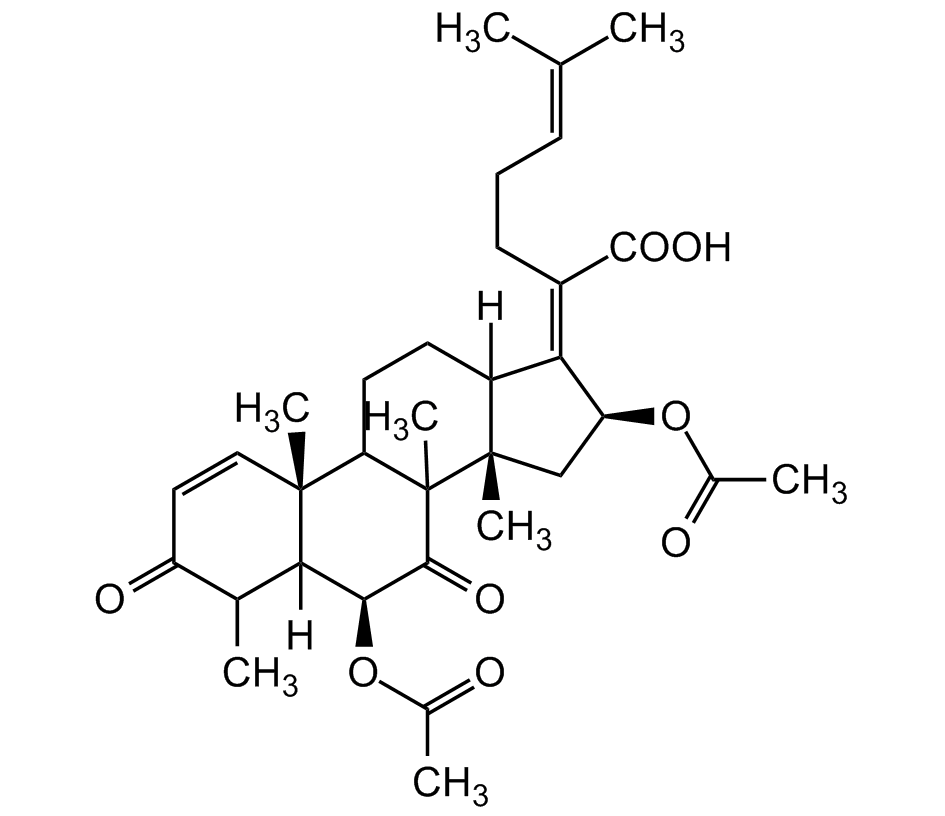
Helvolic acid
| Code | Size | Price |
|---|
| BVT-0435-M001 | 1 mg | £80.00 |
Quantity:
| BVT-0435-M005 | 5 mg | £235.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Fumigacin; NSC 319943; BRN 3230584
Appearance:
Off-white solid.
CAS:
29400-42-8
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302, H319, H332
InChi:
InChI=1S/C33H44O8/c1-17(2)10-9-11-21(30(38)39)26-22-12-13-25-31(6)15-14-23(36)18(3)27(31)28(41-20(5)35)29(37)33(25,8)32(22,7)16-24(26)40-19(4)34/h10,14-15,18,22,24-25,27-28H,9,11-13,16H2,1-8H3,(H,38,39)/b26-21-/t18?,22?,24-,25?,27?,28-,31+,32-,33?/m0/s1
InChiKey:
MDFZYGLOIJNNRM-ADZJIBGLSA-N
Long Description:
Chemical. CAS: 29400-42-8. Formula: C33H44O8. MW: 568.7. Isolated from Aspergillus fumigatus (Strain WDMH-35). Steroidal triterpene. Antibiotic. Antibacterial. Shows broad activity against Gram positive and negative bacteria. Mycotoxin. Antifungal. Antitumor agent. Antituberculosis agent. Oxidized LDL uptake inhibitor. Phytotoxin. Inhibitor of the protein biosynthesis through archeal elongation factor 2 (EF-2).
MDL:
MFCD32689348
Molecular Formula:
C33H44O8
Molecular Weight:
568.7
Package Type:
Plastic Vial
Precautions:
P261, P270, P280, P301, P312, P304, P340
Product Description:
Steroidal triterpene. Antibiotic. Antibacterial. Shows broad activity against Gram positive and negative bacteria. Mycotoxin. Antifungal. Antitumor agent. Cytotoxic against different human cancer cells. Antituberculosis agent. Oxidized LDL uptake inhibitor. Phytotoxin. Inhibitor of the protein biosynthesis through archeal elongation factor 2 (EF-2).
Purity:
>96% (HPLC, NMR)
Signal word:
Warning
SMILES:
[H]C12CCC3[C@@]4(C)C=CC(=O)C(C)C4([H])[C@H](OC(C)=O)C(=O)C3(C)[C@@]1(C)C[C@H](OC(C)=O)C2=C(CCC=C(C)C)C(O)=O
Solubility Chemicals:
Soluble in DMSO, acetone or chloroform.
Source / Host:
Isolated from Aspergillus fumigatus (Strain WDMH-35).
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
The nature of the antibiotic substances produced by Aspergillus fumigatus: S.A. Waksman, et al.; J. Bacteriol. 47, 391 (1944) | Activity of helvolic acid against Mycobacterium tuberculosis: M.A. Jennings; Nature 156, 633 (1945) | Mold metabolites VIII. Contribution to the eluciation of the structure of helvolic acid: D.J. Cram, et al.; JACS 20, 5275 (1956) | Revised structure of helvolic acid: S. Iwasaki, et al.; Chem. Commun. 1970, 1119 (1970) | Inhibition of oxidized low-density lipoprotein metabolism in macrophage J774 by helvolic acid: K.H. Shinohara, & A. Endo; Biochim. Biophys. Acta 1167, 303 (1993) | Isolation and phytotoxic effects of helvolic acid from plant pathogenic fungus Sarocladium oryzae: J.S.M. Tschen, et al.; Bot. Bull. Acad. Sin. 38, 251 (1997) | Fusidic and helvolic acid inhibition of elongation factor 2 from the archaeon Sulfolobus solfataricus: E. De Vendittis, et al.; 41, 14879 (2002) | Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine derived fungus Aspergillus sydowi: M. Zhang, et. al.; J. Nat. Prod. 71, 985 (2008) | Protostadienol biosynthesis and metabolism in the pathogenic fungus Aspergillus fumigatus: S. Lodeiro, et al.; Org. Lett. 11, 1241 (2009) | In vitro synergistic antibacterial activities of helvolic acid on multi-drug resistant Staphylococcus aureus: L. Qin, et al.; Nat. Prod. Res. 23, 309 (2009) | Antimicrobial metabolites from the endophytic fungus Pichia guilliermondii isolated from Paris polyphylla var. yunnanensis: J. Zhao, et al.; Molecules 15, 7961 (2010) | Culture condition-dependent metabolite profiling of Aspergillus fumigatus with antifungal activity: D. Kang, et al.; Fungal Biol. 117, 211 (2013) | Synergistic antitumor efficacy of antibacterial helvolic acid from Cordyceps taii and cyclophosphamide in a tumor mouse model: J.H. Xiao, et al.; Exp. Biol. Med. 242, 214 (2017) | Antituberculosis compounds from a deep-sea-derived fungus Aspergillus sp. SCSIO Ind09F01: X. Lou, et al.; Nat. Prod. Res. 31, 1958 (2017)
Related Products
| Product Name | Product Code | Supplier | Rubrofusarin | BVT-0395 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|