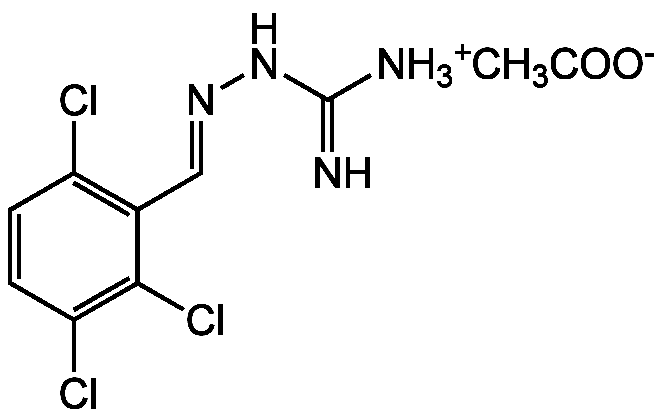
Chloroguanabenz . acetate
| Code | Size | Price |
|---|
| AG-MR-C0036-M001 | 1 mg | £75.00 |
Quantity:
| AG-MR-C0036-M005 | 5 mg | £225.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
1-[(E)-2,3,6-Trichlorophenylmethyleneamino]guanidine; Hydrazinecarboximidamide, 2-[(2,3,6-trichlorophenyl)methylene]-; Guanabenz; Chlorine-Guanabenz
Appearance:
White to off-white powder.
CAS:
23113-55-5
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
After reconstitution, prepare aliquots and store at -20°C.
Hazards:
H301
InChi:
InChI=1S/C8H7Cl3N4.C2H4O2/c9-5-1-2-6(10)7(11)4(5)3-14-15-8(12)13;1-2(3)4/h1-3H,12H2,(H2,13,15);1H3,(H,3,4)/q+1;/p-1/b14-3-;
InChiKey:
DFBSKPLTAXXMPH-LAJPFITQSA-M
Long Description:
Chemical. CAS: 23113-55-5. Formula: C8H7Cl3N4 . C2H4O2. MW: 265.5 . 60.1. Antiprion agent. Inhibitor of protein aggregation. Specifically reduces accumulation of a pathogenic fragment of Huntingtin in a transiently transfected cellular model of Huntington?s disease (HD). Tool for treating HD and other polyglutamine expansion associated diseases.
Molecular Formula:
C8H7Cl3N4 . C2H4O2
Molecular Weight:
265.5 . 60.1
Package Type:
Vial
PG:
III
Precautions:
P270, P301, P310
Product Description:
Antiprion agent. Inhibitor of protein aggregation. Specifically reduces accumulation of a pathogenic fragment of Huntingtin in a transiently transfected cellular model of Huntington?s disease (HD). Tool for treating HD and other polyglutamine expansion associated diseases.
Purity:
>95% (NMR)
Signal word:
Danger
SMILES:
CC(=N)NN=CC1=C(Cl)C=CC(Cl)=C1Cl
Solubility Chemicals:
Soluble in DMSO or ethanol.
Transportation:
Excepted Quantity
UN Nummer:
UN 2811
UNSPSC Category:
Protein Kinase Modulators
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Antihypertensive drug guanabenz is active in vivo against both yeast and mammalian prions: D. Tribouillard-Tanvier, et al.; PLoS One 3, e1981 (2008) | Use of chlorine guanabenz derivatives for treating polyglutamine expansion associated diseases: A. Bertolotti & M. Blondel; Patent WO002008041133A2 (2008) | Synthesis of conjugates of 6-aminophenanthridine and guanabenz, two structurally unrelated prion inhibitors, for the determination of their cellular targets by affinity chromatography: F. Gug, et al.; Bioconjug. Chem. 21, 279 (2010)
Related Products
| Product Name | Product Code | Supplier | 6-Aminophenanthridine | AG-MR-C0029 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6-Amino-8-trifluoromethylphenanthridine | AG-MR-C0031 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||