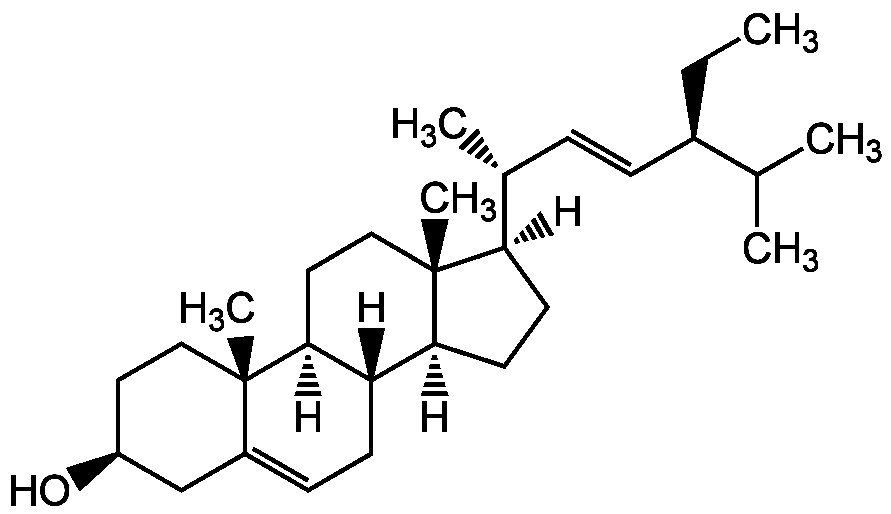
Stigmasterol
Product Code: AG-CN2-0412
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0412-G001 | 1 g | £45.00 |
Quantity:
| AG-CN2-0412-G005 | 5 g | £150.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Stigmasterin; Phytosterol; beta-Stigmasterol; Serposterol; Wulzen anti-stiffness Factor; NSC8095
Appearance:
White to off-white solid.
CAS:
83-48-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C29H48O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h8-10,19-21,23-27,30H,7,11-18H2,1-6H3/b9-8+/t20-,21-,23+,24+,25-,26+,27+,28+,29-/m1/s1
InChiKey:
HCXVJBMSMIARIN-PHZDYDNGSA-N
Long Description:
Chemical. CAS: 83-48-7. Formula: C29H48O. MW: 412.7. Synthetic. Originally isolated from various plants and marine organisms. Anti-hypercholestrolemic compound. Anti-inflammatory and immune-modulating effects. Anticancer compound. Chemopreventive. Cytostatic. Cell growth inhibitor. Antimutagenic. Potent in vitro antagonist of FXR (farnesoid X receptor). DNA polymerase beta inhibitor. Potent antioxidant, hypoglycemic and thyroid inhibiting agent. Anti-osteoarthritic. Decreases the expression of matrix metalloproteinases. Neuroprotective. Is used as a precursor for synthetic progesterone and vitamin D3 and is an intermediate in the biosynthesis of androgens, estrogens and corticoids.
MDL:
MFCD00003630
Molecular Formula:
C29H48O
Molecular Weight:
412.7
Package Type:
Vial
Product Description:
Anti-hypercholestrolemic compound [1, 7]. Anti-inflammatory and immune-modulating effects [2, 4]. Anticancer compound. Chemopreventive [3, 12, 13]. Cytostatic. Cell growth inhibitor [5]. Antimutagenic [6]. Potent in vitro antagonist of FXR (farnesoid X receptor) [8]. DNA polymerase beta inhibitor [9]. Potent antioxidant, hypoglycemic and thyroid inhibiting agent [10]. Anti-osteoarthritic. Decreases the expression of matrix metalloproteinases [11]. Neuroprotective [15]. Is used as a precursor for synthetic progesterone and vitamin D3 and is an intermediate in the biosynthesis of androgens, estrogens and corticoids [14].
Purity:
>95% (NMR)
SMILES:
[H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)C=C[C@@H](CC)C(C)C
Solubility Chemicals:
Soluble in DMSO, ethanol or dimethylformamide. Sparingly soluble in water.
Source / Host:
Synthetic. Originally isolated from various plants and marine organisms.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Antihypercholesterolemic studies with sterols: beta-sitosterol and stigmasterol: R.F. Chandler, et al.; J. Pharm. Sci. 68, 245 (1979) | Topical antiinflammatory activity of phytosterols isolated from Eryngium foetidum on chronic and acute inflammation models: M.D. Garcia, et al.; Phytother. Res. 13, 78 (1999) | Phytosterols as anticancer dietary components: evidence and mechanism of action: A.B. Awad & C.S. Fink; J. Nutr. 130, 2127 (2000) (Review) | Anti-Inflammatory and Immunomodulating Properties of a Sterol Fraction from Sideritis foetens CLEM: N. Antonio, et al.; Biol. Pharm. Bull. 24, 470 (2001) | Cyostatic activity of Achillea ageratum L.: M.A. Gomez, et al.; Phytother. Res. 15, 633 (2001) | Antimutagenic Constituents from the Thorns of Gleditsia sinensis: L. Jae-Chul, et al.; Chem. Pharm. Bull. 53, 561 (2005) | Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat: A.K. Batta, et al.; Metabolism 55, 292 (2006) | Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR: B.A. Carter, et al.; Pediatr. Res. 62, 301 (2007) | Inhibitors of DNA polymerase beta: Activity and mechanism: G. Zhijie, et al.; Bioorg. Med. Chem. 16, 4331 (2008) | Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma: S. Panda, et al.; Fitoterapia 80, 123 (2009) | Stigmasterol: a phytosterol with potential anti-osteoarthritic properties: O. Gabay, et al.; Osteoarthritis Cartilage 18, 106 (2010) | Cytotoxic and apoptotic effects of the oxidized derivatives of stigmasterol in the U937 human monocytic cell line: Y.C. O'Callaghan, et al.; J. Agric. Food Chem. 58, 10793 (2010) | Phytosterols: perspectives in human nutrition and clinical therapy: S.P. Choudhary & L.S. Tran; Curr. Med. Chem. 18, 4557 (2011) (Review) | Stigmasterol: A comprehensive review: N. Kaur, et al.; IJPSR 2, 2259 (2011) (Review) | Rhinacanthus nasutus Extracts Prevent Glutamate and Amyloid-beta Neurotoxicity in HT-22 Mouse Hippocampal Cells: Possible Active Compounds Include Lupeol, Stigmasterol and beta-Sitosterol: J.M. Brimson, et al.; Int. J. Mol. Sci. 13, 5074 (2012)