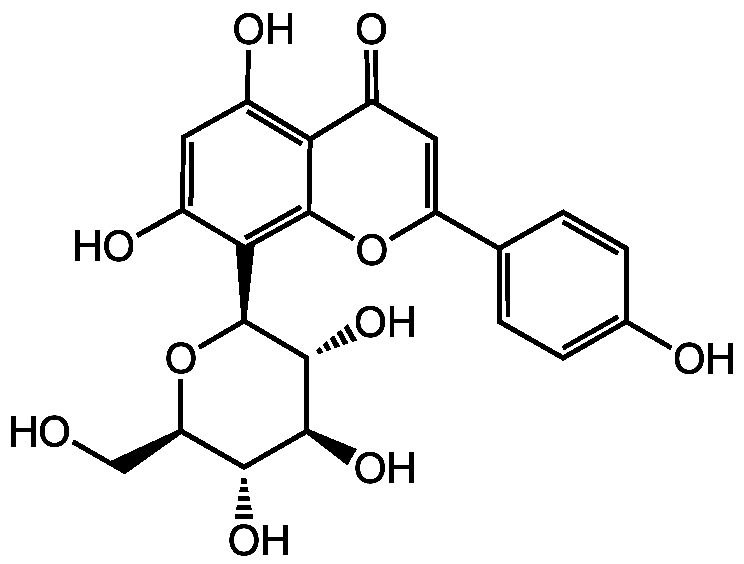
Vitexin
Product Code: AG-CN2-0425
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0425-M005 | 5 mg | £35.00 |
Quantity:
| AG-CN2-0425-M025 | 25 mg | £95.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+20°C
Images
Further Information
Alternate Names/Synonyms:
8-Glucopyranosylapigenin; 8-Glucosylapigenin; Orientoside
Appearance:
Yellow crystalline powder.
CAS:
3681-93-4
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.
InChi:
InChI=1S/C21H20O10/c22-7-14-17(27)18(28)19(29)21(31-14)16-11(25)5-10(24)15-12(26)6-13(30-20(15)16)8-1-3-9(23)4-2-8/h1-6,14,17-19,21-25,27-29H,7H2/t14-,17-,18+,19-,21+/m1/s1
InChiKey:
SGEWCQFRYRRZDC-VPRICQMDSA-N
Long Description:
Chemical. CAS: 3681-93-4. Formula: C21H20O10. MW: 432.4. Isolated from Crataegus pinnatifida. alpha-Glucosidase inhibitor. Antioxidant. HIF-1 alpha inhibitor. Anti-metastatic. Apoptosis inducer. Tumor suppressor. Adipogenesis inhibitor. Inhibits SIRT6 in vitro. Inhibits polyubiquitin synthesis by the ubiquitin-conjugating enzyme E2-25K. Cardioprotective. Anti-inflammatory. Shows analgesic effect by targeting TRPV1 channel activity. Neuroprotective. Inhibits NMDA receptors.
MDL:
MFCD00017456
Molecular Formula:
C21H20O10
Molecular Weight:
432.4
Package Type:
Vial
Product Description:
alpha-Glucosidase inhibitor [3, 8]. Antioxidant [1, 3]. HIF-1 alpha inhibitor [2]. Anti-metastatic [2, 13]. Apoptosis inducer [4, 9, 13]. Tumor suppressor [4, 9]. Adipogenesis inhibitor [5]. Inhibits SIRT6 in vitro [6]. Inhibits polyubiquitin synthesis by the ubiquitin-conjugating enzyme E2-25K [7]. Cardioprotective [10]. Anti-inflammatory [11]. Shows analgesic effect by targeting TRPV1 channel activity [11]. Neuroprotective. Inhibits NMDA receptors [12].
Purity:
>98% (HPLC)
SMILES:
OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)C1=C2OC(=CC(=O)C2=C(O)C=C1O)C1=CC=C(O)C=C1
Solubility Chemicals:
Soluble in DMSO or dimethylformamide.
Source / Host:
Isolated from Crataegus pinnatifida.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
The isolation and antioxidative effects of vitexin from Acer palmatum: J.H. Kim, et al.; Arch. Pharm. Res. 28, 195 (2005) | Vitexin, an HIF-1alpha inhibitor, has anti-metastatic potential in PC12 cells: H.J. Choi, et al.; Mol. Cells 22, 291 (2006) | Antioxidant constituents in the dayflower (Commelina communis L.) and their alpha-glucosidase-inhibitory activity: M. Shibano, et al.; J. Nat. Med. 62, 349 (2008) | Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth: Y. Zhou, et al.; Clin. Cancer Res. 15, 5161 (2009) | Vitexin, orientin and other flavonoids from Spirodela polyrhiza inhibit adipogenesis in 3T3-L1 cells: J. Kim, et al.; Phytother. Res. 24, 1543 (2010) | Synthesis and characterization of SIRT6 protein coated magnetic beads: identification of a novel inhibitor of SIRT6 deacetylase from medicinal plant extracts: M. Yasuda, et al.; Anal. Chem. 83, 7400 (2011) | Vitexin inhibits polyubiquitin synthesis by the ubiquitin-conjugating enzyme E2-25K: K.M. Helms, et al.; Nat. Prod. Commun. 6, 1411 (2011) | Vitexin and isovitexin from the Leaves of Ficus deltoidea with in-vivo alpha-glucosidase inhibition: C.Y. Choo, et al.; J. Ethnopharmacol. 142, 776 (2012) | Apoptosis triggered by vitexin in U937 human leukemia cells via a mitochondrial signaling pathway: C.Y. Lee, et al.; Oncol. Rep. 28, 1883 (2012) | Vitexin protects against cardiac hypertrophy via inhibiting calcineurin and CaMKII signaling pathways: C.C. Lu, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 386, 747 (2013) | Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines: S.M. Borghi, et al.; J. Nat. Prod. 76, 1141 (2013) | The novel p53-dependent metastatic and apoptotic pathway induced by vitexin in human oral cancer OC2 cells: S.H. Yang, et al.; Phytother. Res. 27, 1154 (2013) | Neuroprotective effects of vitexin by inhibition of NMDA receptors in primary cultures of mouse cerebral cortical neurons: L. Yang, et al.; Mol. Cell Biochem. 386, 251 (2014) | A review on the pharmacological effects of vitexin and isovitexin: M. He, et al.; Fitoterapia 115, 74 (2016) | Molecular targets of vitexin and isovitexin in cancer therapy: a critical review: K. Ganesan & B. Xu; Ann. N. Y. Acad. Sci. 1401, 102 (2017) | Vitexin induces apoptosis by suppressing autophagy in multi-drug resistant colorectal cancer cells: M. Bhardwaj, et al.; Oncotarget 9, 3278 (2018)
Related Products
| Product Name | Product Code | Supplier | Wedelolactone | AG-CN2-0424 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Itaconic acid | AG-CN2-0426 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||