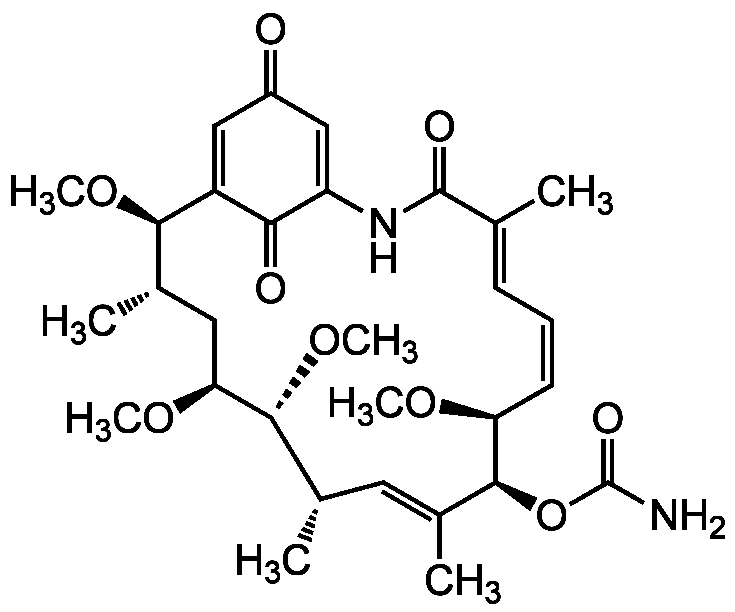
Herbimycin A
Product Code: AG-CN2-0429
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0429-C100 | 100 ug | £140.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Antibiotic TAN 420F; (15R)-17-Demethoxy-15-methoxy-11-O-methyl-geldanamycin
Appearance:
Yellow solid.
CAS:
70563-58-5
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.Protect from light when in solution.
InChi:
InChI=1S/C30H42N2O9/c1-16-10-9-11-23(37-5)28(41-30(31)36)18(3)12-17(2)27(40-8)24(38-6)13-19(4)26(39-7)21-14-20(33)15-22(25(21)34)32-29(16)35/h9-12,14-15,17,19,23-24,26-28H,13H2,1-8H3,(H2,31,36)(H,32,35)/b11-9-,16-10+,18-12+/t17-,19-,23?,24?,26?,27?,28-/m0/s1
InChiKey:
MCAHMSDENAOJFZ-JZYYMJJSSA-N
Long Description:
Chemical. CAS: 70563-58-5. Formula: C30H42N2O9. MW: 574.7. Isolated from Streptomyces sp. Benzoquinone ansamycin antibiotic. Herbicidal compound. Potent, selective, irreversible and cell permeable protein tyrosine kinase inhibitor. Inhibitor of v-Src, Yes, Fps, Ros, Bcr-Abl and ErbB oncogene products. Antitumor compound. Antiangiogenic. Inhibits NF-kappaB activation and phosphorylation of phospholipase C-gamma1. Inhibitor of heat shock protein 90 (Hsp90). Binds Hsp90 and destabilizes client proteins leading to their ubiquitination and proteasomal degradation. Increases the sensitivity of certain cancer cells to chemotherapeutic agents. Neuroprotective. Antiparasitic, antischistosomal agent. Antiviral. Increase microtubules sensitivity to cold in plants.
MDL:
MFCD00151702
Molecular Formula:
C30H42N2O9
Molecular Weight:
574.7
Package Type:
Vial
Product Description:
Benzoquinone ansamycin antibiotic. Herbicidal compound. Potent, selective, irreversible and cell permeable protein tyrosine kinase inhibitor. Inhibitor of v-Src, Yes, Fps, Ros, Bcr-Abl and ErbB oncogene products. Antitumor compound. Antiangiogenic. Inhibits NF-kappaB activation and phosphorylation of phospholipase C-gamma1. Inhibitor of heat shock protein 90 (Hsp90). Binds Hsp90 and destabilizes client proteins leading to their ubiquitination and proteasomal degradation. Increases the sensitivity of certain cancer cells to chemotherapeutic agents. Neuroprotective. Antiparasitic, antischistosomal agent. Antiviral. Increase microtubules sensitivity to cold in plants.
Purity:
>99% (HPLC)
SMILES:
CO[C@H]1C[C@H](C)[C@@H](OC)C2=CC(=O)C=C(NC(=O)C(C)=CC=C/[C@H](OC)[C@@H](OC(N)=O)C(C)=C[C@H](C)[C@H]1OC)C2=O
Solubility Chemicals:
Soluble in ethanol, methanol DMSO or dimethylformamide. Poorly soluble in water.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. Working aliquots are stable for up to 3 months when stored at -20°C. After reconstitution protect from light at -20°C.
References
Herbimycin, a new antibiotic produced by a strain of Streptomyces: S. Omura, et al.; J. Antibiot. (Tokyo) 32, 255 (1979) | A new activity of herbimycin A: inhibition of angiogenesis: T. Yamashita, et al.; J. Antibiot. (Tokyo) 42, 1015 (1989) | Effects of herbimycin A and various SH-reagents on p60v-src kinase activity in vitro: H. Fukazawa, et al; BBRC 173, 276 (1990) | Use and selectivity of herbimycin A as inhibitor of protein-tyrosine kinases: Y. Uehara & H. Fukazawa; Methods Enzymol. 201, 370 (1991) | Specific inhibition of cytoplasmic protein tyrosine kinases by herbimycin A in vitro: H. Fukazawa, et al.; Biochem. Pharmacol. 42, 1661 (1991) | Induction of differentiation of human leukemia cells with a structurally altered c-abl (bcr/abl) gene by herbimycin A, an inhibitor of tyrosine kinase activity: Y. Honma, et al.; Leukemia 6, 229 (1992) | Effect of herbimycin A, an inhibitor of tyrosine kinase, on protein tyrosine kinase activity and phosphotyrosyl proteins of Ph1-positive leukemia cells: M. Okabe, et al.; Leuk. Res. 18, 213 (1994) | Evidence for direct modification of NF kappa B by the tyrosine kinase inhibitor, herbimycin A: T.M. Mahon & L.A. O'Neill; Biochem. Soc. Trans. 23, 111S (1995) | Herbimycin A induces the 20 S proteasome- and ubiquitin-dependent degradation of receptor tyrosine kinases: L. Sepp-Lorenzino, et al.; J. Biol. Chem. 270, 16580 (1995) | Inhibition of PDGF-induced phospholipase D but not phospholipase C activation by herbimycin A: B.Y. Kim; BBRC 212, 1061 (1995) | Specific induction of the 70-kD heat stress proteins by the tyrosine kinase inhibitor herbimycin-A protects rat neonatal cardiomyocytes. A new pharmacological route to stress protein expression? S.D. Morris, et al.; J. Clin. Invest. 97, 706 (1996) | Inhibition of PDGF-induced phospholipase C activation by herbimycin A: B.Y. Kim, et al.; Biochim. Biophys. Acta 1311, 33 (1996) | Herbimycin A enhances apoptotic effect of chemotherapeutic drugs on K562 cells: H. Ren, et al.; Chin. Med. J. (Engl.) 111, 678 (1998) | Hsp-90-associated oncoproteins: multiple targets of geldanamycin and its analogs: M.V. Blagosklonny; Leukemia 16, 455 (2002) (Review) | Herbimycin A induces sympathetic neuron survival and protects against hypoxia: M.C. Cabral-da-Silva, et al.; Neuroreport 14, 2397 (2003) | Natural product origins of Hsp90 inhibitors: Y. Uehara; Curr. Cancer Drug Targets 3, 325 (2003) | Herbimycin A abrogates nuclear factor-kappaB activation by interacting preferentially with the IkappaB kinase beta subunit: S. Ogino, et al.; Mol. Pharmacol. 65, 1344 (2004) | c-Src is required for complex formation between the hepatitis C virus-encoded proteins NS5A and NS5B: a prerequisite for replication: A. Pfannkuche, et al.; Hepatology 53, 1127 (2011) | Inhibitors of tyrosine kinases and phosphatases as a tool for the investigation of microtubule role in plant cold response: Y.A. Sheremet, et al.; Tsitol Genet. 46, 3 (2012) | Transcriptome analyses of inhibitor-treated schistosome females provide evidence for cooperating Src-kinase and TGFbeta receptor pathways controlling mitosis and eggshell formation: C. Buro, et al.; PLoS Pathog. 9, e1003448 (2013)
Related Products
| Product Name | Product Code | Supplier | Genistein | AG-CN2-0427 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|