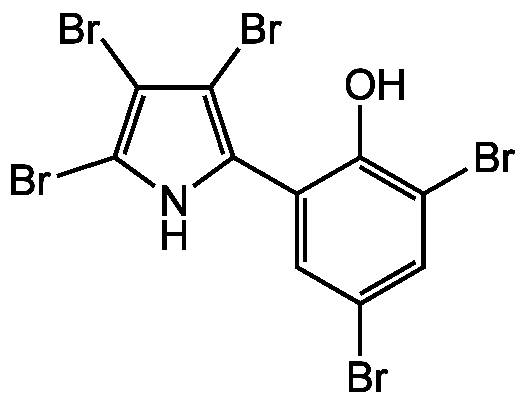
Pentabromopseudilin
| Code | Size | Price |
|---|
| BVT-0441-C500 | 500 ug | £180.00 |
Quantity:
| BVT-0441-M001 | 1 mg | £310.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
BI
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Pentabrompseudilin; NSC641543; NSC288032; PBQ; 2-(3,5-Dibromo-2-hydroxyphenyl)-3,4,5-tribromopyrrol; 2,4-Dibromo-6-(3,4,5-tribromo-1H-pyrrol-2-yl)phenol
Appearance:
Off-white powder.
CAS:
10245-81-5
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light.
Hazards:
H302, H312, H319
InChi:
InChI=1S/C10H4Br5NO/c11-3-1-4(9(17)5(12)2-3)8-6(13)7(14)10(15)16-8/h1-2,16-17H
InChiKey:
LXMNWKJHYOZUQL-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 10245-81-5. Formula: C10H4Br5NO. MW: 553.7. Isolated from sea water proteobacteria Alteromonas Iuteo-violaceus. Antibiotic. Antibacterial, cytotoxic, herbicidal and antimalarial compound. Human lipoxygenase inhibitor. Potent myosin ATPase inhibitor. Inhibitor of the non-mevalonate pathway enzyme IspD.
MDL:
MFCD32067414
Molecular Formula:
C10H4Br5NO
Molecular Weight:
553.7
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P302, P352, P312
Product Description:
Antibiotic [1, 2, 4, 6]. Antibacterial, cytotoxic, herbicidal and antimalarial compound [3, 9, 11, 13]. Human lipoxygenase inhibitor [7]. Potent myosin ATPase inhibitor [8, 10, 14]. Inhibitor of the non-mevalonate pathway enzyme IspD [13].
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
OC1=C(Br)C=C(Br)C=C1C1=C(Br)C(Br)=C(Br)N1
Solubility Chemicals:
Soluble in DMSO (5mg/ml), acetone, butanol, ethylacetate or diethylether.
Source / Host:
Isolated from sea water proteobacteria Alteromonas Iuteo-violaceus.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
Production of a pyrrole antibiotic by a marine bacterium: P.R. Burkholder, et al.; Appl. Microbiol. 14, 649 (1966) | The structure of a bromine-rich marine antibiotic: F.M. Lovell; JACS 88, 4510 (1966) | Marine bacteria. I. Synthesis of pentabromopseudilin, a cytotoxic phenylpyrrole from Alteromonas luteoviolaceus: H. Laatsch & H. Pudleiner; Liebigs Ann. Chem. 9, 863 (1989) | Biosynthesis of the marine antibiotic pentabromopseudilin. Part 1. The benzene ring: U. Hanefeld, et al.; J. Org. Chem. 8, 847 (1993) | Structure-activity relationships of phenyl- and benzoylpyrroles: H. Laatsch, et al.; Chem. Pharm. Bull. 43, 537 (1995) | Biosynthesis of the marine antibiotic pentabromopseudilin. Part 2. The pyrrole ring: J. Peschke, et al.; Biosci. Biotechnol. Biochem. 69, 628 (2005) | A rev(V)-catalysed C-N bond-forming route to human lipoxygenase inhibitors: O. Rachana, et al.; Org. Lett. 7, 2501 (2005) | The mechanism of pentabromopseudilin inhibition of myosin motor activity: R. Fedorov, et al.; Nat. Struct. Mol. Biol. 16, 80 (2009) | Highly brominated antimicrobial metabolites from a marine Pseudoalteromonas sp.: D. Feher, et al.; J. Nat. Prod. 73, 1963 (2010) | Inhibition of myosin ATPase activity by halogenated pseudilins: a structure-activity study: M. Preller, et al.; J. Med. Chem. 54, 3675 (2011) | Agrochemicals against malaria, sleeping sickness, leishmaniasis and chagas Disease: M. Witschel, et al.; PLoS Negl. Trop. Dis. 6, 1805 (2012) | Small-molecule inhibitors of myosin proteins: L.M. Bond, et al.; Future Med. Chem. 5, 41 (2013) | Toxicity of bioactive and probiotic marine bacteria and their secondary metabolites in Artemia sp. and Caenorhabditis elegans as eukaryotic model organisms: A.K. Neu, et al.; Appl. Environ. Microbiol. 80, 146 (2014) | Pseudilins: halogenated, allosteric inhibitors of the non-mevalonate pathway enzyme IspD: A. Kunfermann, et al.; Angew. Chem. Int. Ed. Engl. 53, 2235 (2014) | Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation: E. Rozbicki, et al.; Nat. Cell Biol. 17, 397 (2015)