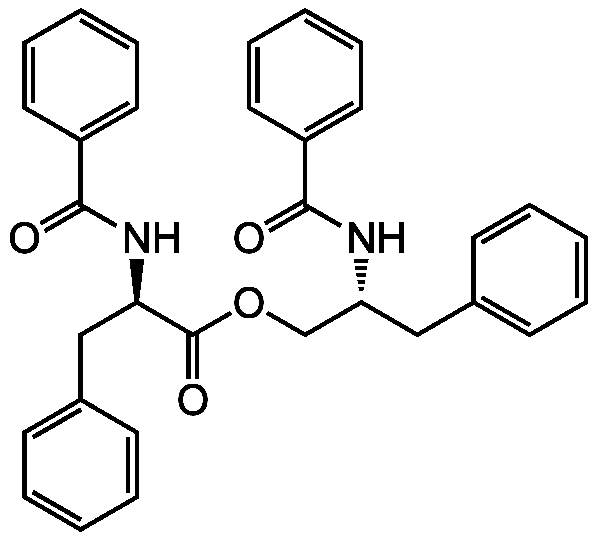
Asperphenamate
Product Code: AG-CN2-0171
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0171-C250 | 250 ug | £90.00 |
Quantity:
| AG-CN2-0171-M001 | 1 mg | £250.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Anabellamide; Asjanin; Auranamide; NSC 306231; N-Benzoyl-phenylalanine-2-benzoylamino-3-phenylpropyl ester
Appearance:
White solid.
CAS:
63631-36-7
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Hazards:
H302
InChi:
InChI=1S/C32H30N2O4/c35-30(26-17-9-3-10-18-26)33-28(21-24-13-5-1-6-14-24)23-38-32(37)29(22-25-15-7-2-8-16-25)34-31(36)27-19-11-4-12-20-27/h1-20,28-29H,21-23H2,(H,33,35)(H,34,36)/t28-,29-/m1/s1
InChiKey:
CVULDJMCSSACEO-FQLXRVMXSA-N
Long Description:
Chemical. CAS: 63631-36-7. Formula: C32H30N2O4. MW: 506.6. Isolated from Aspergillus sp. Anticancer compound. Cytotoxic against human breast cancer cells. Induces autophagic cell death in MCF-7 cells. Moderate radical scavenger. Weak acetylcholinesterase (AChE) inhibitor. Shows moderate trypanocidal activity.
MDL:
MFCD30180097
Molecular Formula:
C32H30N2O4
Molecular Weight:
506.6
Package Type:
Vial
Precautions:
P270, P301, P312, P330
Product Description:
Anticancer compound. Cytotoxic against human breast cancer cells. Induces autophagic cell death in MCF-7 cells. Moderate radical scavenger. Weak acetylcholinesterase (AChE) inhibitor. Shows moderate trypanocidal activity.
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
O=C(OC[C@@H](CC1=CC=CC=C1)NC(=O)C1=CC=CC=C1)[C@@H](CC1=CC=CC=C1)NC(=O)C1=CC=CC=C1
Solubility Chemicals:
Soluble in ethanol, methanol or DMSO.
Source / Host:
Isolated from Aspergillus sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Two metabolites from Aspergillus flavipes: A.M. Clark, et al.; Lloyda 40, 146 (1977) | Cytotoxic and anti-HIV principles from the rhizomes of Begonia nantoensis: P.W. Wu, et al.; Chem. Pharm. Bull. 52, 345 (2004) | A new method for asperphenamate synthesis and its antimicrobial activity evaluation: A.M. Pomini, et al.; Nat. Prod. Res. 20, 537 (2006) | Total synthesis and anticancer activity studies of the stereoisomers of asperphenamate and patriscabratine: L. Yuane, et al.; Chin. Chem. Lett. 21, 155 (2010) | JNK-dependent Atg4 upregulation mediates asperphenamate derivative BBP-induced autophagy in MCF-7 cells: Y. Li, et al.; Toxicol. Appl. Pharmacol. 263, 21 (2012) | Two new Penicillium species Penicillium buchwaldii and Penicillium spathulatum, producing the anticancer compound asperphenamate: J.C. Frisvad, et al.; FEMS Microbiol. Lett. 339, 77 (2013) | In vitro acetylcholinesterase activity of peptide derivatives isolated from two species of Leguminosae: C.Q. Alves, et al.; Pharm. Biol. 51, 936 (2013) | Antioxidant activity of compounds isolated from the root woods of Erythrina droogmansiana: A.J.G. Yaya, et al.; Int. J. Pharm. Sci. Drug Res. 6, 160 (2014) | Two trypanocidal dipeptides from the roots of Zapoteca portoricensis (Fabaceae): N.J. Nwodo, et al.; Molecules 19, 5470 (2014)