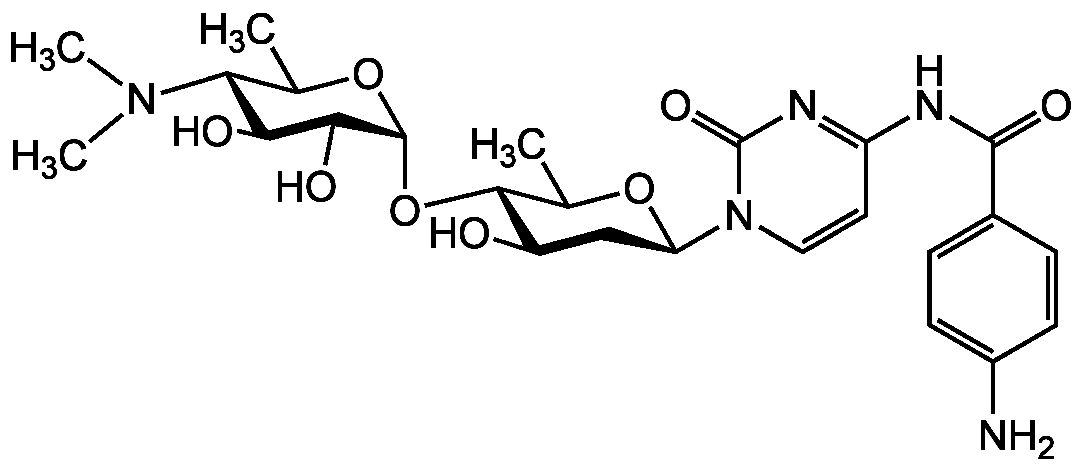
Oxyplicacetin
| Code | Size | Price |
|---|
| BVT-0031-M001 | 1 mg | £130.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
3'-Hydroxyplicacetin; Cytosaminomycin E
Appearance:
White solid.
CAS:
100108-92-7
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light when in solution.
Hazards:
H302, H319
InChi:
InChI=1S/C25H35N5O8/c1-12-19(29(3)4)20(32)21(33)24(37-12)38-22-13(2)36-18(11-16(22)31)30-10-9-17(28-25(30)35)27-23(34)14-5-7-15(26)8-6-14/h5-10,12-13,16,18-22,24,31-33H,11,26H2,1-4H3,(H,27,28,34,35)/t12-,13-,16-,18-,19-,20+,21-,22-,24-/m1/s1
InChiKey:
UYEYXSOGZWDEFY-LJEPMGMESA-N
Long Description:
Chemical. CAS: 100108-92-7. Formula: C25H35N5O8. MW: 533.6. Isolated from Streptomyces sp. Nucleoside antibiotic (amicetin group). Anticoccidial agent. Shows broad antibacterial activity.
MDL:
N/A
Molecular Formula:
C25H35N5O8
Molecular Weight:
533.6
Package Type:
Plastic Vial
Precautions:
P270, P280, P301, P312, P305, P351, P338
Product Description:
Nucleoside antibiotic (amicetin group). Anticoccidial agent. Shows broad antibacterial activity.
Purity:
>98% (NMR)
Signal Word:
Warning
SMILES:
CC1O[C@H](C[C@@H](O)[C@@H]1O[C@H]1OC(C)[C@H]([C@H](O)C1O)N(C)C)N1C=CC(NC(=O)C2=CC=C(N)C=C2)=NC1=O
Solubility Chemicals:
Soluble in DMSO or water.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C.
References
Studies on metabolites produced by Streptomyces ramulosus Tue-34. II. The structural elucidation of oxyplicacetin, a new amicetin: Y. Chen, et al.; Kangshengsu 10, 285 (1985) (Chinese) | Cytosaminomycins, new anticoccidial agents produced by Streptomyces sp. KO-8119. I. Taxonomy, production, isolation and physico-chemical and biological properties: K. Haneda, et al.; J. Antibiot. 47, 774 (1994) | Cytosaminomycins, new anticoccidial agents produced by Streptomyces sp. KO-8119. II. Structure elucidation of cytosaminomycins A, B, C and D: K. Shiomi, et al.; J. Antibiot. 47, 782 (1994) | Characterization of the amicetin biosynthesis gene cluster from Streptomyces vinaceusdrappus NRRL 2363 implicates two alternative strategies for amide bond formation: G. Zhang, et al.; Appl. Environ. Microbiol. 78, 2393 (2012)