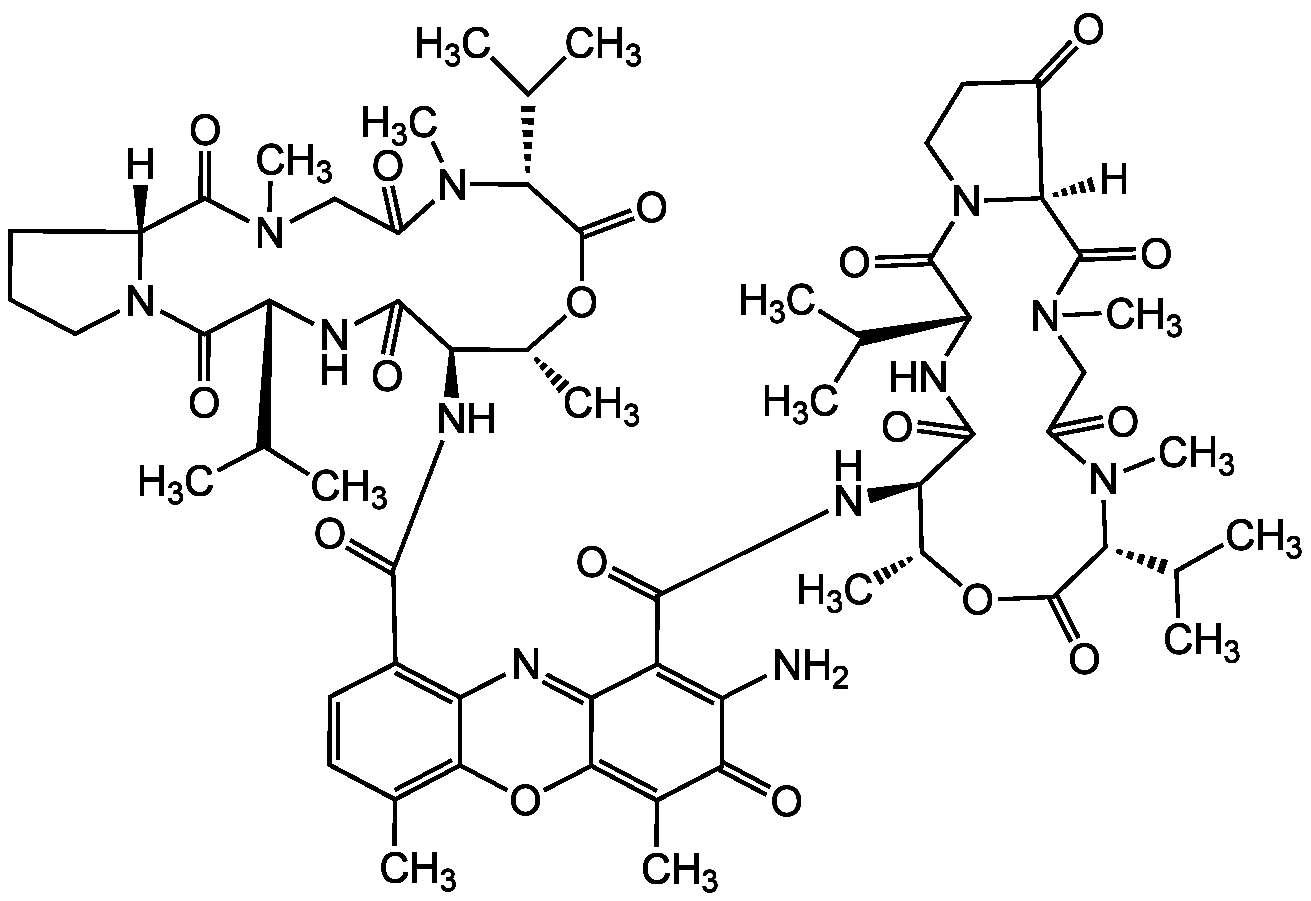
Actinomycin X2
| Code | Size | Price |
|---|
| BVT-0375-M001 | 1 mg | £105.00 |
Quantity:
| BVT-0375-M005 | 5 mg | £300.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Actinomycin A5; Actinomycin B2; Actinomycin V; Actinomycin D, 3A-(4-oxo-L-proline); NSC175006
Appearance:
Orange crystals.
CAS:
18865-48-0
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
After reconstitution protect from light at -20°C.
Hazards:
H302, H312, H332
InChi:
InChI=1S/C62H84N12O17/c1-26(2)41-58(84)73-22-17-18-35(73)57(83)69(13)24-37(76)71(15)47(28(5)6)61(87)89-32(11)43(55(81)65-41)67-53(79)34-20-19-30(9)51-45(34)64-46-39(40(63)50(78)31(10)52(46)91-51)54(80)68-44-33(12)90-62(88)48(29(7)8)72(16)38(77)25-70(14)60(86)49-36(75)21-23-74(49)59(85)42(27(3)4)66-56(44)82/h19-20,26-29,32-33,35,41-44,47-49H,17-18,21-25,63H2,1-16H3,(H,65,81)(H,66,82)(H,67,79)(H,68,80)/t32-,33-,35-,41-,42-,43-,44-,47-,48-,49+/m1/s1
InChiKey:
SMBASHZPOHFQRU-ILYIJYFNSA-N
Long Description:
Chemical. CAS: 18865-48-0. Formula: C62H84N12O17. MW: 1269.4. Isolated from Streptomyces sp. Aniso-Actinomycin (4-Oxopro instead of Pro in the beta-chain). Antitumor and antiviral antibiotic. Antituberculosis agent. Has higher cytotoxicity towards cultured human leukemia (HL-60) cells than actinomycin D. Apoptosis inducer. Forms a complex with DNA and blocks its biological activity.
MDL:
MFCD00076115
Molecular Formula:
C62H84N12O17
Molecular Weight:
1269.4
Package Type:
Plastic Vial
Precautions:
P261, P301, P312, P302, P352, P304, P340
Product Description:
Aniso-Actinomycin (4-Oxopro instead of Pro in the beta-chain). Antitumor and antiviral antibiotic. Antituberculosis agent. Has higher cytotoxicity towards cultured human leukemia (HL-60) cells than actinomycin D. Apoptosis inducer. Forms a complex with DNA and blocks its biological activity.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
[H][C@]12CCCN1C(=O)[C@H](NC(=O)[C@H](NC(=O)C1=C3N=C4C(OC3=C(C)C=C1)=C(C)C(=O)C(N)=C4C(=O)N[C@@H]1[C@@H](C)OC(=O)[C@@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@]3([H])N(CCC3=O)C(=O)[C@H](NC1=O)C(C)C)[C@@H](C)OC(=O)[C@@H](C(C)C)N(C)C(=O)CN(C)C2=O)C(C)C
Solubility Chemicals:
Soluble in DMSO, methanol, acetone or chloroform.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
Actinomycin V as a Potent Differentiation Inducer of F5-5 Friend Leukemia Cells: H. Morioka, et al.; Agric. Biol. Chem. 49, 2835 (1985) | Structure elucidation of a potent anti-MRSA antibiotic AM3, produced by Streptomyces sp.: Y. Lim, et al.; Agric. Chem. Biotech. 38, 516 (1995) | Identification of endophytic Streptomyces sp. R-5 and analysis of its antimicrobial metabolites: M. Shimizu, et al.; J. Gen. Plant Pathol. 70, 66 (2004) | The actinomycins: Anticancer agents from natural products: A. B. Mauger & H. Lackner; page 281, CRC Press (2005) | Characterization of Streptomyces MITKK-103, a newly isolated Actinomycin X2-producer: K. Kurosawa, et al.; Appl. Microbiol. Biotechnol. 72, 145 (2006) | A marine-derived Streptomyces sp. MS449 produces high yield of actinomycin X2 and actinomycin D with potent anti-tuberculosis activity: C. Chen, et al.; Appl. Microbiol. Biotechnol. 95, 919 (2012) | Characterization of Streptomyces padanus JAU4234, a producer of actinomycin X2, fungichromin, and a new polyene macrolide antibiotic: Z.-Q. Xiong, et al.; Appl. Environ. Microbiol. 78, 589 (2012) | Purification and structure determination of three bioactive molecules from a newly isolated Streptomyces sp. AH 47: A. Hamza, et al.; Int. J. Curr. Res. 5, 1548 (2013) | Apoptosis of human prostate cancer cells induced by marine actinomycin X2 through the mTOR pathway compounded by MiRNA144: J. Liu, et al.; Anticancer Drugs 27, 156 (2016) | Identification, Bioactivity, and Productivity of Actinomycins from the Marine-Derived Streptomyces heliomycini: D. Wang, et al.; Front. Microbiol. 8, 1147 (2017)