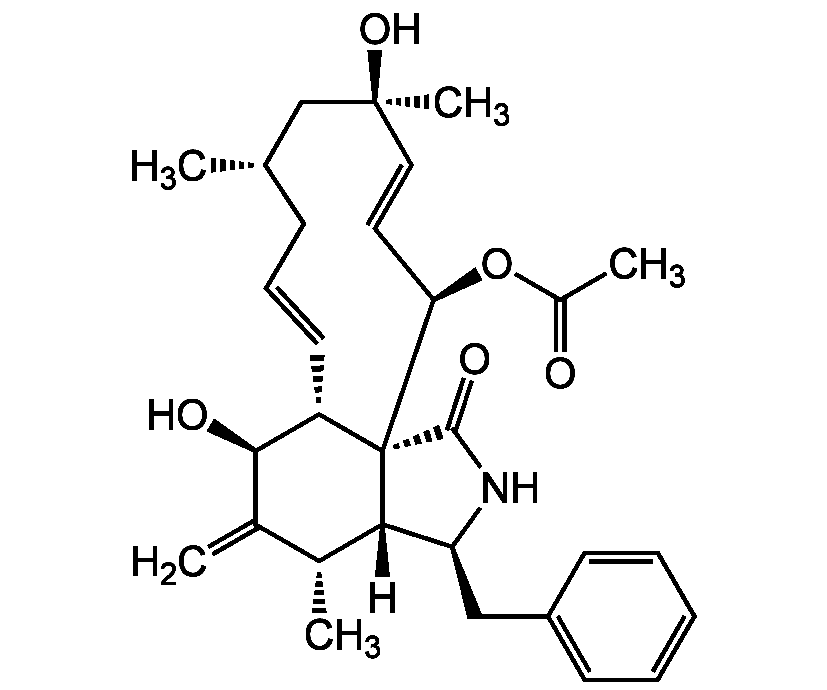
Cytochalasin H
| Code | Size | Price |
|---|
| BVT-0447-M001 | 1 mg | £110.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Paspalin P1; Kodocytochalasin 1; Cytochalasin O; NSC305222; 17-Deoxo-21-acetylzygosporin D
Appearance:
White solid.
CAS:
53760-19-3
Class:
6.1
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS06
Handling Advice:
After reconstitution protect from light at -20°C.
Hazards:
H300, H310, H330, H361
InChi:
InChI=1S/C30H39NO5/c1-18-10-9-13-23-27(33)20(3)19(2)26-24(16-22-11-7-6-8-12-22)31-28(34)30(23,26)25(36-21(4)32)14-15-29(5,35)17-18/h6-9,11-15,18-19,23-27,33,35H,3,10,16-17H2,1-2,4-5H3,(H,31,34)/b13-9+,15-14-/t18-,19+,23-,24-,25+,26-,27+,29-,30+/m0/s1
InChiKey:
NAEWXXDGBKTIMN-CGCBHLOZSA-N
Long Description:
Chemical. CAS: 53760-19-3. Formula: C30H39NO5. MW: 493.6. Isolated from Phomopsis sp. Potent mycotoxin. Phytotoxin. Actin polymerization inhibitor. Used in actin polymerization and cytoskeletal reorganization studies. Antibacterial, antifungal, nematocidal and antitumor compound. Apoptosis inducer. Anti-angiogenic agent. Shows immunosuppressive activity. Shown to modulate the CNS with potential anti-parkinson activity.
MDL:
MFCD00010539
Molecular Formula:
C30H39NO5
Molecular Weight:
493.6
Package Type:
Plastic Vial
PG:
II
Precautions:
P301, P310, P302, P350, P304, P340
Product Description:
Potent mycotoxin. Phytotoxin. Actin polymerization inhibitor. Used in actin polymerization and cytoskeletal reorganization studies. Antibacterial, antifungal, nematocidal and antitumor compound. Apoptosis inducer. Anti-angiogenic agent. Shows immunosuppressive activity. Shown to modulate the CNS with potential anti-parkinson activity.
Purity:
>98% (HPLC, NMR)
Signal Word:
Danger
SMILES:
[H][C@]12[C@H](CC3=CC=CC=C3)NC(=O)[C@@]11[C@@H](C=CC[C@H](C)C[C@@](C)(O)C=C[C@H]1OC(C)=O)[C@H](O)C(=C)[C@H]2C
Solubility Chemicals:
Soluble in DMSO, methanol or acetone.
Source / Host:
Isolated from Phomopsis sp.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
Toxicity and plant growth regulator effects of cytochalasin H isolated from Phomopsis sp.: J.M. Wells, et al.; Can. J. Microbiol. 22, 1137 (1976) | Structure of a new [11]cytochalasin, cytochalasin H or kodo-cytochalasin-1: M. A. Beno, et al.; JACS 99, 4123 (1977) | Effect of cytochalasins B & H on isoelectric focusing of chick embryonic neural retina cells: S. Ghaskadbi & L. Mulherkar; Indian J. Exp. Biol. 20, 869 (1982) | Effects of cytochalasin H on chick embryo explants cultured in vitro: S. Ghaskadbi, et al. Toxicology 33, 323 (1984) | Effects of cytochalasin and phalloidin on actin: J.A. Cooper; J. Cell Biol. 105, 1473 (1987) | A new immunosuppressive cytochalasin isolated from a Pestalotia sp.: N. S. Burres, et al.; J. Antibiot. 45, 1367 (1992) | Effects of cytochalasin H, a potent inhibitor of cytoskeletal reorganization, on platelet function: P. Natajaran, et al.; Platelets, 11, 467 (2000) | 1H and 13C NMR assignments of three nitrogen containing compounds from the mangrove endophytic fungus (ZZF08): Y. Tao, et al.; Magn. Reson. Chem. 46, 501 (2008) | Cytotoxic cytochalasin metabolites of endophytic Endothia gyrosa: S. Xu, et al.; Chem. Biodivers. 6, 739 (2009) | Characterization of kinase suppressor of Ras-1 expression and anticancer drug sensitivity in human cancer cell lines: S.M. Stoeger & K.H. Cowan; Cancer Chemother. Pharmacol. 63, 807 (2009) | Application of cytochalasin compound cytochalasin H in manufacture of the medicine for resisting Parkinson's disease: L. Shet, et al.; CN103816149 A20140528 (2014) (Patent) | Cytochalasin H, an active anti-angiogenic constituent of the ethanol extract of Gleditsia sinensis Thorns: J. Lee, et al.; Biol. Pharm. Bull. 37, 6 (2014)