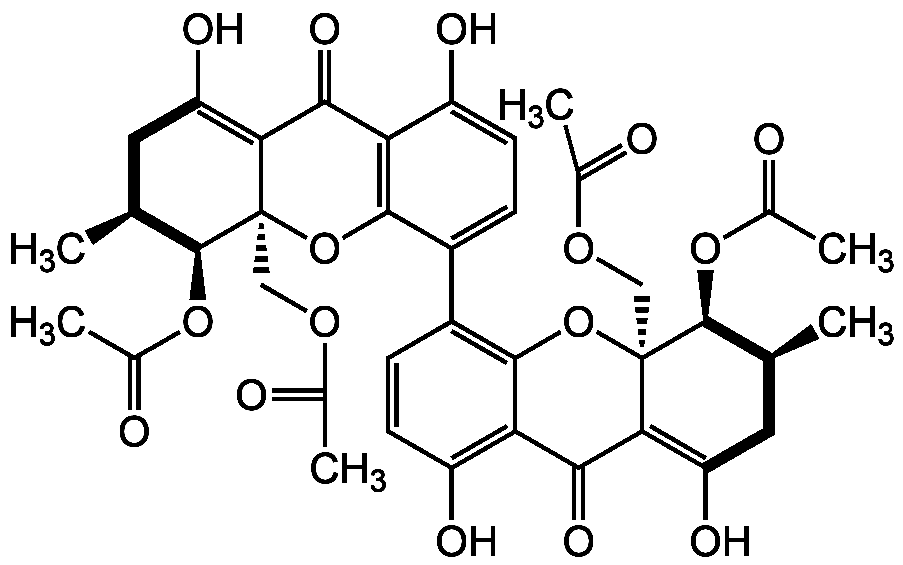
Phomoxanthone A
| Code | Size | Price |
|---|
| BVT-0453-M005 | 5 mg | £350.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Appearance:
Pale yellow solid.
CAS:
359844-69-2
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light when in solution.
InChi:
InChI=1S/C38H38O16/c1-15-11-25(45)29-31(47)27-23(43)9-7-21(33(27)53-37(29,13-49-17(3)39)35(15)51-19(5)41)22-8-10-24(44)28-32(48)30-26(46)12-16(2)36(52-20(6)42)38(30,54-34(22)28)14-50-18(4)40/h7-10,15-16,35-36,43-46H,11-14H2,1-6H3/t15-,16-,35-,36-,37+,38+/m0/s1
InChiKey:
OHHXJWHRQGZQJM-CMDKCIDSSA-N
Long Description:
Chemical. CAS: 359844-69-2. Formula: C38H38O16. MW: 750.7. Isolated from fungus Phomopsis sp M4. Mycotoxin Antibacterial against Gram-positive bacteria. Shows antimalarial and antitubercular activity. Anticancer compound. Cytotoxic against several cancer cell lines (pro-apoptotic activity). Active against plant pathogene fungi (e.g. P. oryza, Phyt. infestans). Activator of murine T lymphocytes, NK cells and macrophages.
MDL:
MFCD28899334
Molecular Formula:
C38H38O16
Molecular Weight:
750.7
Package Type:
Plastic Vial
Product Description:
Mycotoxin Antibacterial against Gram-positive bacteria. Shows antimalarial and antitubercular activity. Anticancer compound. Cytotoxic against several cancer cell lines (pro-apoptotic activity). Active against plant pathogene fungi (e.g. P. oryza, Phyt. infestans). Activator of murine T lymphocytes, NK cells and macrophages. Mitochondrial toxin with a mode of action distinct from known electron transport chain (ETC) inhibitors, OXPHOS uncouplers and ionophores. Shows rapid inhibition of both ETC and DeltaPsim, the release of mitochondrial Ca2+ and fission of the inner but not the outer mitochondrial membrane independent from the mitochondrial fission and fusion regulators DRP1 and OPA1.
Purity:
>97% (1H-NMR, HPLC)
SMILES:
C[C@H]1CC(O)=C2C(=O)C3=C(O[C@@]2(COC(C)=O)[C@H]1OC(C)=O)C(=CC=C3O)C1=C2O[C@@]3(COC(C)=O)[C@@H](OC(C)=O)[C@@H](C)CC(O)=C3C(=O)C2=C(O)C=C1
Solubility Chemicals:
Soluble in DMSO, methanol or acetone. Insoluble in water.
Source / Host:
Isolated from fungus Phomopsis sp M4.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C.
References
Phomoxanthones A and B, novel xanthone dimers from the endophytic fungus Phomopsis species: M. Isaka, et al.; J. Nat. Prod. 64, 1015 (2001) | X-ray Structure Determination, Absolute Configuration and Biological Activity of Phomoxanthone A: B. Elsasser, et al.; Eur. J. Org. Chem. 2005, 4563 (2005) | Anticancer compounds derived from fungal endophytes: their importance and future challenges: R.N. Kharwar, et al.; Nat. Prod. Rep. 28, 1208 (2011) | Isolation of a phomoxanthone A derivative, a new metabolite of tetrahydroxanthone, from a Phomopsis sp. isolated from the mangrove, Rhizhhopora mucronata: Y. Shiono, et al.; Nat. Prod. Commun. 8, 1735 (2013) | Pro-apoptotic and immunostimulatory tetrahydroxanthone dimers from the endophytic fungus Phomopsis longicolla: D. Rönsberg, et al.; J. Org. Chem. 78, 12409 (2013) | The mycotoxin phomoxanthone A disturbs the form and function of the inner mitochondrial membrane: P. Boehler, et al.; Cell Death Dis. 9, 286 (2018)