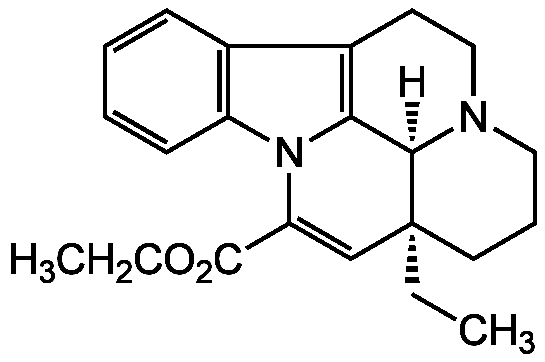
Vinpocetine
Product Code: AG-CN2-0454
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0454-M020 | 20 mg | £45.00 |
Quantity:
| AG-CN2-0454-M100 | 100 mg | £70.00 |
Quantity:
| AG-CN2-0454-G001 | 1 g | £105.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short term storage:+20°C. Long term storage:+20°C.
Images
Further Information
Alternate Names/Synonyms:
VPC; (3alpha,16alpha)-Eburnamenine-14-carboxylic acid ethyl ester; Ethyl apovincaminate; RGH-4405; TCV 3B; AY 27255
Appearance:
White to slightly yellow crystalline powder.
CAS:
42971-09-5
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H302, H319, H335
InChi:
InChI=1S/C22H26N2O2/c1-3-22-11-7-12-23-13-10-16-15-8-5-6-9-17(15)24(19(16)20(22)23)18(14-22)21(25)26-4-2/h5-6,8-9,14,20H,3-4,7,10-13H2,1-2H3/t20-,22+/m1/s1
InChiKey:
DDNCQMVWWZOMLN-IRLDBZIGSA-N
Long Description:
Chemical. CAS: 42971-09-5. Formula: C22H26N2O2. MW: 350.5. Semi-synthetic from Voacanga africana. Selective Ca2+-calmodulin dependent cGMP-phosphodiesterase (PDE1) inhibitor. Shows vasorelaxant activity. Neuroprotective agent. Selectively inhibits voltage-sensitive 2+ channels Potent anti-inflammatory agent. Inhibitor of NF-kappaB-dependent inflammatory responses by directly targeting IKK. Shown to inhibit the NLRP3 inflammasome. Antioxidant. Free radical scavenger. Anticancer compound. Anticonvulsant.
MDL:
MFCD00211233
Molecular Formula:
C22H26N2O2
Molecular Weight:
350.5
Package Type:
Vial
Precautions:
P261, P264, P270, P271, P280, P301, P312
Product Description:
Selective Ca2+-calmodulin dependent cGMP-phosphodiesterase (PDE1) inhibitor. Shows vasorelaxant activity. Neuroprotective agent. Selectively inhibits voltage-sensitive Na2+ channels Potent anti-inflammatory agent. Inhibitor of NF-kappaB-dependent inflammatory responses Inhibitor of IkappaB kinase IKK. Shown to inhibit the NLRP3 inflammasome. Antioxidant. Free radical scavenger. Anticancer compound. Anticonvulsant. Attenuates osteoblastic differentiation of vascular smooth muscle cells. Anti-schistosomal agent. Attenuates liver fibrosis
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
CC[C@@]12CCCN3[C@@H]1C4=C(CC3)C5=CC=CC=C5N4C(=C2)C(=O)OCC
Solubility Chemicals:
Soluble in DMSO, dichloromethane, acetone or 100% ethanol. Insoluble in water.
Source / Host:
Semi-synthetic from Voacanga africana.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +20°C.
References
Effects of selective inhibitors on cyclic nucleotide phosphodiesterases of rabbit aorta: H.S. Ahn, et al.; Biochem. Pharmacol. 38, 3331 (1989) | Role of selective cyclic GMP phosphodiesterase inhibition in the myorelaxant actions of M&B 22,948, MY-5445, vinpocetine and 1-methyl-3-isobutyl-8-(methylamino)xanthine: J.E. Souness, et al.; Br. J. Pharmacol. 98, 725 (1989) | Vinpocetine selectively inhibits neurotransmitter release triggered by sodium channel activation: M. Sitges & V. Nekrassov; Neurochem. Res. 24, 1585 (1999) | In vitro antioxidant properties of pentoxifylline, piracetam, and vinpocetine: B. Horvath, et al.; Clin. Neuropharmacol. 25, 37 (2002) | Effects of Vinpocetine on mitochondrial function and neuroprotection in primary cortical neurons: K. Tarnok, et al.; Neurochem. Int. 53, 289 (2008) | Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine - a PDE1 inhibitor: R. Deshmukh, et al.; Eur. J. Pharmacol. 620, 49 (2009) | Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism: K.I. Jeon, et al.; PNAS 107, 9795 (2010) | Vinpocetine as a potent antiinflammatory agent: A.E. Medina, et al.; PNAS 107, 9921 (2010) | Vinpocetine inhibits breast cancer cells growth in vitro and in vivo: E.W. Huang, et al.; Apoptosis 17, 1120 (2012) | Vinpocetine attenuates lipid accumulation and atherosclerosis formation: Y. Cai, et al.; BBRC 434, 439 (2013) | The anti-seizure drugs vinpocetine and carbamazepine, but not valproic acid, reduce inflammatory IL-1beta and TNF-alpha expression in rat hippocampus: C.D. Gomez, et al.; J. Neurochem. 130, 770 (2014) | Anti-inflammatory effects of vinpocetine in atherosclerosis and ischemic stroke: a review of the literature: L. Zhang & L. Yang; Molecules 20, 335 (2014) | Vinpocetine inhibits amyloid-beta induced activation of NF-kappaB, NLRP3 inflammasome and cytokine production in retinal pigment epithelial cells: R.T. Liu, et al.; Exp. Eye Res. 127, 49 (2014) | Vinpocetine modulates metabolic activity and function during retinal ischemia: L. Nivison-Smith, et al.; Am. J. Physiol. Cell Physiol. 308, 737 (2015) | Vinpocetine reduces lipopolysaccharide-induced inflammatory pain and neutrophil recruitment in mice by targeting oxidative stress, cytokines and NF-kappaB: K.W. Ruiz-Miyazawa, et al.; Chem. Biol. Interact. 237, 9 (2015) | Vinpocetine attenuates osteoblastic differentiation of vascular smooth muscle cells: Y.Y. Ma, et al.; PLoS One 11, e0162295 (2016) | A comparative study on the anti-schistosomal and hepatoprotective effects of vinpocetine and isosorbide-5-mononitrate on Schistosoma mansoni-infected mice: S.M. Alhusseiny, et al.; Acta Tropica 176, 114 (2017) | Novel action of vinpocetine in the prevention of paraquat-induced parkinsonism in mice: involvement of oxidative stress and neuroinflammation: I.O. Ishola, et al.; Metab. Brain Dis. 35, 1493 (2018) | The reduction of XIAP is associated with inflammasome activation in RPE: implications for AMD pathogenesis: J. Gao, et al.; J. Neuroinflamm. 16, 171 (2019) | Vinpocetine attenuates thioacetamide-induced liver fibrosis in rats: A.E. Elnfarawy, et al. Hum. Exp. Toxicol. ahead of print, 2020 | Updates of Recent Vinpocetine Research in Treating Cardiovascular Diseases: Ch. Zhang & Ch. Yan; J. Cell Immunol. 2, 211 (2020) (Review) | Vinpocetine protects against the development of experimental abdominal aortic aneurysms: Ch. Zhang, et al.; Clin. Sci. 134, 2959 (2020)
Related Products
| Product Name | Product Code | Supplier | Shikonin | AG-CN2-0487 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|