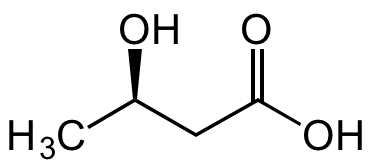
(R)-3-Hydroxybutyric acid
| Code | Size | Price |
|---|
| AG-CR1-3616-M025 | 25 mg | £50.00 |
Quantity:
| AG-CR1-3616-M100 | 100 mg | £90.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Antibody Isotype: n/a
Antibody Clone: n/a
Regulatory Status: RUO
Shipping:
+4°C
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(R)-beta-Hydroxybutyric acid; D-(-)-3-Hydroxybutyric acid; D-(-)-BHB
Appearance:
White solid.
CAS:
625-72-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C4H8O3/c1-3(5)2-4(6)7/h3,5H,2H2,1H3,(H,6,7)/t3-/m1/s1
InChiKey:
WHBMMWSBFZVSSR-GSVOUGTGSA-N
Long Description:
Chemical. CAS: 625-72-9. Formula: C4H8O3. MW: 104.1. Key metabolite of the ketolytic pathway generating acetyl-CoA. Stereoselective product of D-3-hydroxybutyrate dehydreogenase. Clinically and physiologically significant stereoisomer. Energy carrier from adipocytes to peripheral tissues during fasting or exercise. Endogenous inhibitor of histone deacetylases (HDACs) 1, 3 and 4. Ligand of free fatty acid receptor 3 (FFAR3; GPR41) and hydroxycarboxylic acid receptor 2 (HCAR2; GPR109B). NLRP3 inflammasome inhibitor. Prevents K+-efflux and reduces ASC oligomerization and speck formation.
MDL:
MFCD00066257
Molecular Formula:
C4H8O3
Molecular Weight:
104.1
Other data:
Optical Rotation: [alpha]20/D c=6 in H2O: -25°?1.0°
Package Type:
Vial
Precautions:
P261, P271, P280, P312
Product Description:
Key metabolite of the ketolytic pathway generating acetyl-CoA. Stereoselective product of D-3-hydroxybutyrate dehydreogenase. Clinically and physiologically significant stereoisomer. Energy carrier from adipocytes to peripheral tissues during fasting or exercise. Endogenous inhibitor of histone deacetylases (HDACs) 1, 3 and 4. Ligand of free fatty acid receptor 3 (FFAR3; GPR41) and hydroxycarboxylic acid receptor 2 (HCAR2; GPR109B). NLRP3 inflammasome inhibitor. Prevents K+-efflux and reduces ASC oligomerization and speck formation.
Purity:
>98% (Assay)
Signal word:
Warning
SMILES:
C[C@@H](O)CC(O)=O
Solubility Chemicals:
Soluble in water, 100% ethanol or methanol.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Effects of beta-hydroxy butyric acid on insulin binding to its receptor and on autophosphorylation of the receptor: H. Ohtusaka, et al.; Endocrinol. Jpn. 37, 915 (1990) | The direct measurement of 3-beta-hydroxy butyrate enhances the management of diabetic ketoacidosis in children and reduces time and costs of treatment: M. Vanelli, et al.; Diab. Nutr. Metab. 16, 312 (2003) | Detection of cerebral {beta}-hydroxy butyrate, acetoacetate, and lactate on proton MR spectroscopy in children with diabetic ketoacidosis: S.L. Wootton-Gorges, et al.; AJNR 26, 1286 (2005) | beta-Hydroxybutyrate activates the NF-kappaB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes: X. Shi, et al.; Cell. Physiol. Biochem. 33, 920 (2014) | beta-hydroxybutyrate: Much more than a metabolite: J. C. Newman & E. Verdin; Diab. Res. Clin. Pract. 106, 173 (2014) (Review) | BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-kappaB activation: S.P. Fu, et al.; Med. Inflamm. 2014, ID983401 (2014) | Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson's disease models are mediated by GPR109A-dependent mechanisms: S.P. Fu, et al.; J. Neuroinflamm. 12, ID9 (2015) | The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease: Y.H. Youm, et al.; Nat. Med. 21, 263 (2015) | Taming the inflammasome: M. Levy, et al.; Nat. Med. 21, 213 (2015) | Inflammasome inhibition: putting out the fire: M.G. Netea & L.A. Joosten; Cell Metab. 21, 513 (2015) | Inflammasome: starving inflammation: E. Kugleberg; Nat. Rev. Immunol. 15, 199 (2015) (Review) | The Nlrp3 inflammasome admits defeat: C.J. Gross & O. Gross; Trends Immunol. 36, 323 (2015) (Review)
Related Products
| Product Name | Product Code | Supplier |
|---|