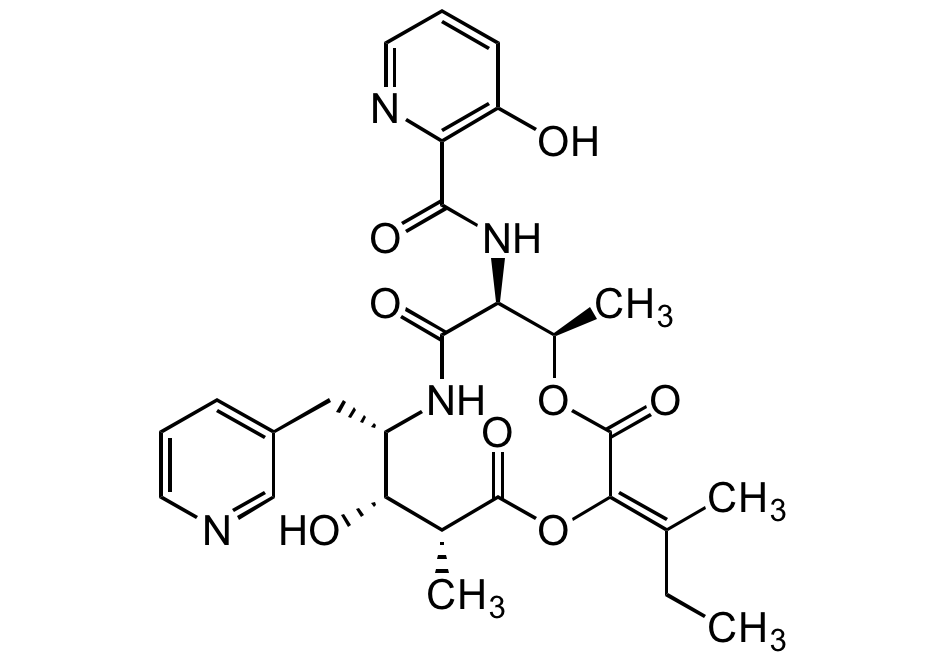
Pyridomycin
| Code | Size | Price |
|---|
| BVT-0455-M001 | 1 mg | £300.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Antibiotic U 24544; Erizomycin; NSC 246134
Appearance:
Off-white solid.
CAS:
18791-21-4
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light when in solution.
InChi:
InChI=1S/C27H32N4O8/c1-5-14(2)23-27(37)38-16(4)20(31-25(35)21-19(32)9-7-11-29-21)24(34)30-18(12-17-8-6-10-28-13-17)22(33)15(3)26(36)39-23/h6-11,13,15-16,18,20,22,32-33H,5,12H2,1-4H3,(H,30,34)(H,31,35)/b23-14-/t15-,16-,18+,20+,22+/m1/s1
InChiKey:
WHIKSLGSXKIHCA-IGCCMALHSA-N
Long Description:
Chemical. CAS: 18791-21-4. Formula: C27H32N4O8. MW: 540.6. Isolated from Streptomyces sp. Cyclodepsipeptide antibiotic. Unusual 12-membered macrocyclic depsipeptide comprises three unique subunits incorporating two substituted pyridines. Potent antitubercular agent. Inhibitor of NADH-dependent enoyl (Acyl-Carrier-Protein) reductase InhA, preventing mycolic acid synthesis in M. tuberculosis. Shown to be active against isoniazid-resistant mycobacteria.
MDL:
MFCD01690307
Molecular Formula:
C27H32N4O8
Molecular Weight:
540.6
Package Type:
Plastic Vial
Product Description:
Cyclodepsipeptide antibiotic. Unusual 12-membered macrocyclic depsipeptide comprises three unique subunits incorporating two substituted pyridines. Potent antitubercular agent. Inhibitor of NADH-dependent enoyl (Acyl-Carrier-Protein) reductase InhA, preventing mycolic acid synthesis in M. tuberculosis. Shown to be active against isoniazid-resistant mycobacteria.
Purity:
>98% (1H-NMR, HPLC)
SMILES:
CCC(C)=C1/OC(=O)[C@H](C)[C@H](O)[C@H](CC2=CC=CN=C2)NC(=O)[C@@H](NC(=O)C2=C(O)C=CC=N2)[C@@H](C)OC1=O
Solubility Chemicals:
Soluble in DMSO, methanol, water, dichloromethane or acetone.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C.
References
A new antibiotic, pyridomycin: K. Maeda, et al.; J. Antibiot. (Tokyo) 6, 140 (1953) | Structure of pyridomycin: G. Koyama, et al.; Tetrahedron Lett. 37, 3587 (1967) | Chemistry of pyridomycin: H. Ogawara, et al.; Chem. Pharm. Bull. 16, 679 (1968) | Synthetic studies of pyridomycin. V. Total synthesis of pyridomycin: M. Kinoshita, et al.; Tetrahedron Lett. 30, 7419 (1989) | Identification and characterization of the pyridomycin biosynthetic gene cluster of streptomyces pyridomyceticus NRRL B-2517: T. Huang, et al.; J. Biol. Chem. 286, 20648 (2011) | Towards a new tuberculosis drug: pyridomycin - nature's isoniazid: R. C. Hartkoorn, et al.; EMBO Mol. Medicine 4, 1032 (2012) | Pyridomycin bridges the NADH- and substrate-binding pockets of the enoyl reductase InhA: R. C. Hartkoorn, et al.; Nat. Chem. Biol. 10, 96 (2014)