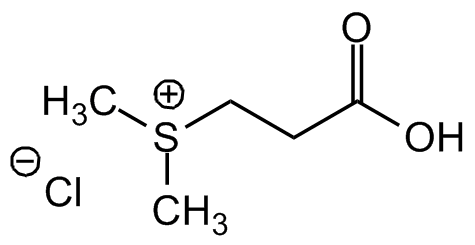
Dimethylsulfoniopropionate . HCl
| Code | Size | Price |
|---|
| BVT-0468-M100 | 100 mg | £140.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
Short Term: +4°C Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
3-Dimethylsulfoniopropionate; Dimethylpropiothetin (DMPT); (2-Carboxyethyl)dimethylsulfonium betaine
Appearance:
White needles. Stable salt form.
CAS:
4337-33-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Hygroscopic.
Hazards:
H315, H335
InChi:
InChI=1S/C5H10O2S.ClH/c1-8(2)4-3-5(6)7;/h3-4H2,1-2H3;1H
InChiKey:
RRUMKKGRKSSZKY-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 4337-33-1. Formula: C5H10O2S . HCl. MW: 134.2 . 36.5. Synthetic. Naturally occurring organosulfur compound. Osmolyte, osmoprotectant. Antioxidant. Cryoprotectant. Neuroprotective agent. Neuritogenic. Acts against immune-deficient diseases (e.g. cancer). Important in geochemistry, ecology and algal/plant physiology. Metabolite produced primarily by marine phytoplankton and is the main precursor to the climatically important gas dimethylsulfide (DMS).
MDL:
MFCD00142888
Molecular Formula:
C5H10O2S . HCl
Molecular Weight:
134.2 . 36.5
Package Type:
Plastic Vial
Precautions:
P261, P271, P280, P304, P340, P305, P351, P338, P405
Product Description:
Naturally occurring organosulfur compound. Osmolyte, osmoprotectant. Antioxidant. Cryoprotectant. Neuroprotective agent. Neuritogenic. Acts against immune-deficient diseases (e.g. cancer). Important in geochemistry, ecology and algal/plant physiology. Chemoattractant for marine planktonic microbes and planktivorous reef fishes. Metabolite produced primarily by marine phytoplankton and is the main precursor to the climatically important gas dimethylsulfide (DMS).
Purity:
>98% (NMR)
Signal Word:
Warning
SMILES:
[Cl-].C[S+](C)CCC(O)=O
Solubility Chemicals:
Soluble in DMSO, methanol or water.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
A precursor of the dimethyl sulphide evolved by Polysiphonia fastigiata. Dimethyl-2-carboxyethylsulphonium hydroxide and its salts: F. Challenger & M.I. Simpson; J. Chem. Soc. 1591 (1948) | An antioxidant function for DMSP and DMS in marine algae: W. Sunda, et al.; Nature 418, 317 (2002) | Direct quantification of dimethylsulfoniopropionate (DMSP) with hydrophilic interaction liquid chromatography/mass spectrometry: A. Spielmeyer & G. Pohnert; J. Chromatogr. B, 878, 3238 (2010) | Novel pathwax for the assimilation of dimethylsulfoniopropionate widespread in marine bacteria: C.R. Reisch, et al.; Nature 473, 208 (2011) | DMSP biosynthesis by an animal and its role in coral thermal stress response: J.-B. Raina, et al.; Nature 502, 677 (2013) | Defence chemistry modulation by light and temperature shifts and the resulting effects on associated epibacteria of Fucus vesiculosus: M. Saha, et al.; PLoS One 9, e105333 (2014) | Amelioration effect of a tertiary sulfonium compound, dimethylsulfoniopropionate, in green sea algae on Ehrlich Ascitic-tumor, solid tumor and related diseases: K. Nakajima; in Handbook Anticancer Drugs from Marine Origin (ed. S.-K. Kim), 205 (2015) | The chemical biology of dimethylsulfoniopropionate: J.S. Dickschat, et al.; Org. Biomol. Chem. 13, 1954 (2015)