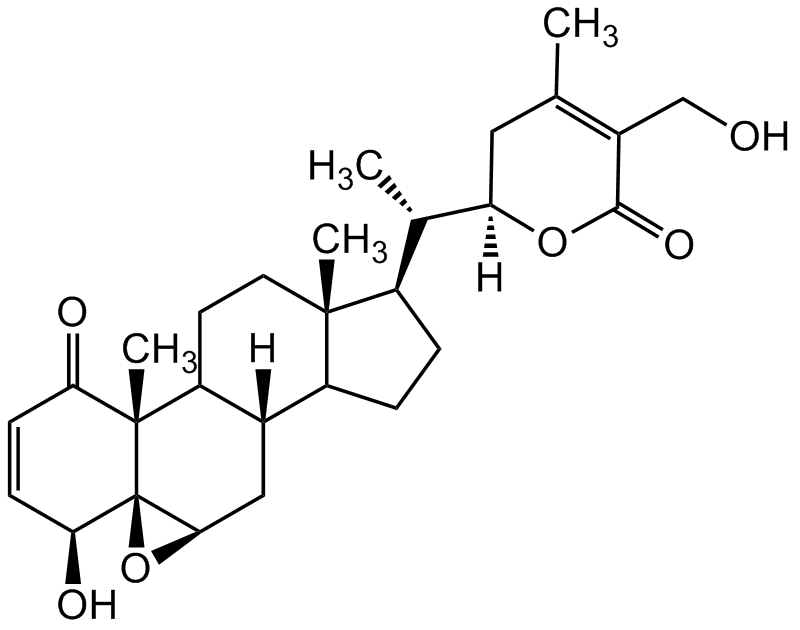
Withaferin A
Product Code: AG-CN2-0490
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0490-M001 | 1 mg | £75.00 |
Quantity:
| AG-CN2-0490-M005 | 5 mg | £205.00 |
Quantity:
| AG-CN2-0490-M010 | 10 mg | £310.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Storage:
Short Term: 4°C Long Term: -20°C
Images
Further Information
Alternate Names/Synonyms:
NSC 101088; NSC 273757; 5beta,6beta-Epoxy-4beta,22R,27-trihydroxy-1-oxo-delta-lactone-ergosta-2,24-dien-26-oic acid
Appearance:
White to off-white solid.
CAS:
5119-48-2
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light when in solution.
InChi:
InChI=1S/C28H38O6/c1-14-11-21(33-25(32)17(14)13-29)15(2)18-5-6-19-16-12-24-28(34-24)23(31)8-7-22(30)27(28,4)20(16)9-10-26(18,19)3/h7-8,15-16,18-21,23-24,29,31H,5-6,9-13H2,1-4H3/t15-,16-,18+,19?,20?,21+,23-,24+,26+,27-,28+/m0/s1
InChiKey:
DBRXOUCRJQVYJQ-KUVJLYTASA-N
Long Description:
Chemical. CAS: 5119-48-2. Formula: C28H38O6. MW: 470.6. Isolated from Withania somnifera. Multifunctional anticancer compound. Amplifies the cellular antioxidant and/or detoxification system, suppresses inflammatory pathways, selectively inhibits tumor cell proliferation and induces apoptosis, suppresses tumor angiogenesis, blocks epithelial-to-mesenchymal transition (EMT), tumor invasion and metastasis, alters tumor cell metabolism, induces immunomodulation and downregulates cancer stem cells targets. Sensitizes resistant cancer cells to existing chemotherapeutic agents. Antioxidant. Induces cytoprotective enzymes (Nrf2-dependent as well), including SOD, HO-1 (heme oxygenase-1), catalase, glutathione peroxidise, glutathione s-transferase and NQO1. Anti-inflammatory agent. Inhibits COX-2 and iNOS expression (at protein and mRNA level) and PGE2 production. Prevents NF-kappaB activation by inhibiting activation of IKKbeta. Inhibits Helicobacter pylori-induced IL-1beta production by regulating NF-kappaB and directly inhibiting NLRP3 inflammasome activation. Antiproliferative agent. Induces G2/M phase cell cycle arrest, targeting CDKs. Shown to modulate STAT3, Bcl2, Notch receptors, Akt, Hsp90, EGFR, HER2 and PCNA. Disrupts the organization of microtubules and actin/microfilament. Stimulates F-actin cross-linking. Induces tumor cell apoptosis. Shown to induce ROS production. Activates mitochondrial apoptosis pathway through Bcl-2 downregulation and Bax upregulation. Shown to induce p53-dependent pathways and ER-stress. Activates the tumor suppressor protein PAR-4. Directly or indirectly inhibits selected other kinases, such MAPK or STAT3. Shown to inhibit hedgehog signalling pathway. Described to be an autophagy inducer and inhibitor. Potent inhibitor of the 20S proteasome beta5 subunit chymotrypsin-like activity. Potent angiogenesis inhibitor by modulating VEGF. Antimigratory, antiinvasive and antimetastatic compound. Targets TGF-beta, vimentin, MMPs and Notch receptors. Inhibits cancer stem cells targets, including aldehyde dehydrogenase 1 (ALDH1) activity. Alters cell line-specific the mRNA expression of cancer stem cell markers, such as Oct4, SOX-2, and Nanog. Reported to induce neuronal regeneration. Antidiabetic compound. Potent Leptin sensitizer, acting similar to Celastrol (Prod. No. AG-CN2-0460).
MDL:
MFCD10687098
Molecular Formula:
C28H38O6
Molecular Weight:
470.6
Package Type:
Vial
Product Description:
Multifunctional anticancer compound. Amplifies the cellular antioxidant and/or detoxification system, suppresses inflammatory pathways, selectively inhibits tumor cell proliferation and induces apoptosis, suppresses tumor angiogenesis, blocks epithelial-to-mesenchymal transition (EMT), tumor invasion and metastasis, alters tumor cell metabolism, induces immunomodulation and downregulates cancer stem cells targets. Sensitizes resistant cancer cells to existing chemotherapeutic agents. Antioxidant. Induces cytoprotective enzymes (Nrf2-dependent as well), including SOD, HO-1 (heme oxygenase-1), catalase, glutathione peroxidise, glutathione s-transferase and NQO1. Anti-inflammatory agent. Inhibits COX-2 and iNOS expression (at protein and mRNA level) and PGE2 production. Prevents NF-kappaB activation by inhibiting activation of IKKbeta. Inhibits Helicobacter pylori-induced IL-1beta production by regulating NF-kappaB and directly inhibiting NLRP3 inflammasome activation. Antiproliferative agent. Induces G2/M phase cell cycle arrest, targeting CDKs. Shown to modulate STAT3, Bcl2, Notch receptors, Akt, Hsp90, EGFR, HER2 and PCNA. Disrupts the organization of microtubules and actin/microfilament. Stimulates F-actin cross-linking. Induces tumor cell apoptosis. Shown to induce ROS production. Activates mitochondrial apoptosis pathway through Bcl-2 downregulation and Bax upregulation. Shown to induce p53-dependent pathways and ER-stress. Activates the tumor suppressor protein PAR-4. Directly or indirectly inhibits selected other kinases, such MAPK or STAT3. Shown to inhibit hedgehog signalling pathway. Described to be an autophagy inducer and inhibitor. Potent inhibitor of the 20S proteasome beta5 subunit chymotrypsin-like activity. Potent angiogenesis inhibitor by modulating VEGF. Antimigratory, antiinvasive and antimetastatic compound. Targets TGF-beta, vimentin, MMPs and Notch receptors. Inhibits cancer stem cells targets, including aldehyde dehydrogenase 1 (ALDH1) activity. Alters cell line-specific the mRNA expression of cancer stem cell markers, such as Oct4, SOX-2, and Nanog. Reported to induce neuronal regeneration. Antidiabetic compound. Potent Leptin sensitizer, acting similar to Celastrol (Prod. No. AG-CN2-0460).
Purity:
>98% (HPLC)
SMILES:
O=C1[C@@]2(C)[C@@]([C@H]3C[C@]4([H])C2CC[C@@]5(C)C4CC[C@@H]5[C@@H]([C@]6([H])OC(C(CO)=C(C)C6)=O)C)(O3)[C@@H](O)C=C1
Solubility Chemicals:
Soluble in DMSO (10mg/ml), DMF, 100% ethanol (5mg/ml), methanol (5mg/ml) or ethyl acetate. Insoluble in water.
Source / Host:
Isolated from Withania somnifera.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352211
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Stock solutions are stable for at least 3 months when stored at -20°C.
References
Antitumor activity of withaferin A (NSC-101088): B. Shohat, et al.; Cancer Chemother. Rep. 51, 271 (1967) | Withaferin A is a potent inhibitor of angiogenesis: R. Mohan, et al.; Angiogenesis 7, 115 (2004) | Neuritic regeneration and synaptic reconstruction induced by withanolide: A: T. Kuboyama, et al.; Br. J. Pharmacol. 144, 961 (2005) | The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from "Indian winter cherry": H. Yang, et al.; Mol. Pharmacol. 71, 426 (2007) | The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin: P. Bargagna-Mohan, etal.; Chem. Biol. 14, 623 (2007) | Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade: C. Mandal, et al.; Apoptosis 13, 1450 (2008) | Inhibition of monosodium urate crystal-induced inflammation by withaferin A: E.P. Sabina, et al.; J. Pharm. Pharm. Sci. 11, 46 (2008) | Withaferin A targets heat shock protein 90 in pancreatic cancer cells: Y. Yu, et al.; Biochem. Pharmacol. 79, 542 (2010) | Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis: S. Koduru, et al.; Mol. Cancer Ther. 9, 202 (2010) | Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells: J. Lee, et al.; Carcinogenesis. 31, 1991 (2010) | Inhibition of the NEMO/IKK? association complex formation, a novel mechanism associated with the NF-kB activation suppression by Withania somnifera's key metabolite withaferin A: A. Grover, et al.; BMC Genomics 11, s25 (2010) | Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2: E. Mayola, et al.; Apoptosis 16, 1014 (2011) | Withaferin a alters intermediate filament organization, cell shape and behavior: B. Grin, et al.; PLoS One 7, e39065 (2012) | Withaferin A causes activation of Notch2 and Notch4 in human breast cancer cells: J. Lee, et al.; Breast Cancer Res. Treat. 136, 45 (2012) | Molecular insight in the multifunctional activities of Withaferin A: W. Van den Berghe, et al.; Biochem. Pharmacol. 84, 1282 (2012) (Review) | Inhibition of VEGF: a novel mechanism to control angiogenesis by Withania somnifera's key metabolite Withaferin A: S. Saha, et al.; In Silico Pharmacol. 1, 11 (2013) | Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKK?: K. Heyninck, et al.; Biochem. Pharmacol. 91, 501 (2014) | Withaferin A Inhibits Helicobacter pylori-induced Production of IL-1beta in Dendritic Cells by Regulating NF-kappaB and NLRP3 Inflammasome Activation: J.E. Kim, et al.; Immune Netw. 15, 269 (2015) | Molecular docking and dynamic simulation studies evidenced plausible immunotherapeutic anticancer property by Withaferin A targeting indoleamine 2,3-dioxygenase: S.V. Reddy, et al.; J. Biomol. Struct. Dyn. 33, 2695 (2015) | Hedgehog inhibitors from Withania somnifera: T. Yoneyama, et al.; Bioorg. Med. Chem. Lett. 25, 3541 (2015) | The Use of Withaferin A to study intermediate filaments: R. Mohan & P. Bargagna-Mohan; Methods Enzymol. 568, 187 (2016) | Simultaneous inhibition of the ubiquitin-proteasome system and autophagy enhances apoptosis induced by ER stress aggravators in human pancreatic cancer cells: Autophagy 12, 1521 (2016) | Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice: J. Lee, et al.; Nat. Med. 22, 1023 (2016) | Withaferin-A - A natural anticancer agent with pleitropic mechanisms of action: I.C. Lee & B.Y. Choi; Int. J. Mol. Sci. 17, 290 (2016) (Review)