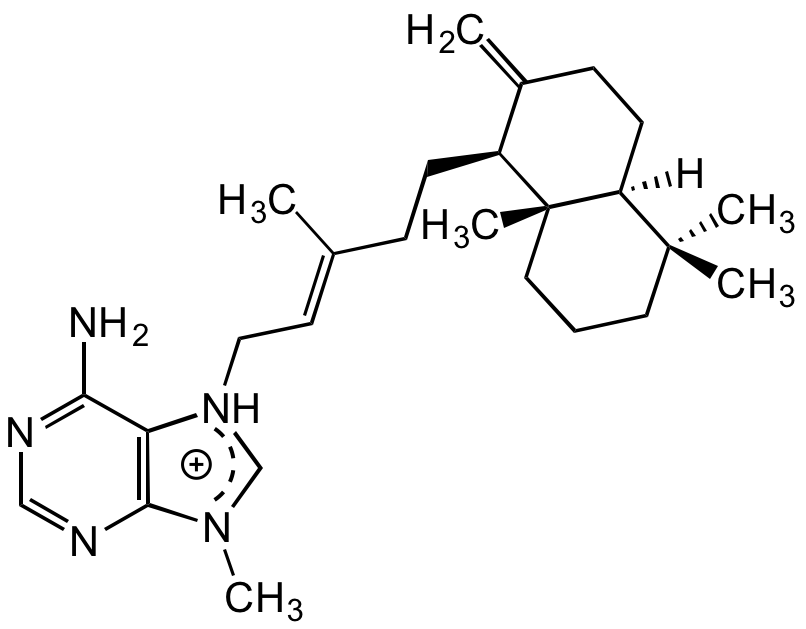
Agelasine D
Product Code: AG-CN2-0492
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0492-M001 | 1 mg | £190.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(+)-Agelasine D
Appearance:
Off-white to colorless crystals.
CAS:
92664-80-7
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep cool and dry.Protect from light.
InChi:
InChI=1S/C26H41N5/c1-18(12-15-31-17-30(6)24-22(31)23(27)28-16-29-24)8-10-20-19(2)9-11-21-25(3,4)13-7-14-26(20,21)5/h12,16,20-21H,2,7-11,13-15,17H2,1,3-6H3,(H2,27,28,29)/p+1/b18-12+/t20-,21-,26+/m0/s1
InChiKey:
SHVHRNDHUVXXIF-XGBZQHFCSA-O
Long Description:
Chemical. CAS: 92664-80-7. Formula: C26H40N5. MW: 422.6. Isolated from sponge Agelas sp. Antifouling compound. Antimycobacterial and antibacterial agent. Exerts its antibacterial effect by inhibiting enzyme BCG 3185c, a suspected dioxygenase thereby disrupting bacterial homeostasis. Associated with contractive responses of smooth muscles and inhibition of Na+/K+ ATPase. Cytotoxic/Antineoplastic agent. Found to exhibit inhibitory activity against several cancer cell lines, including the drug resistant renal cancer cell line (ACHN). Suppressor of RANKL-induced osteoclastogenesis via down-regulation of c-Fos, NFATc1, NF-kappaB and ERK. Antiprotozoal compound.
MDL:
MFCD09264156
Molecular Formula:
C26H40N5
Molecular Weight:
422.6
Package Type:
Vial
Product Description:
Antifouling compound. Antimycobacterial and antibacterial agent. Exerts its antibacterial effect by inhibiting enzyme BCG 3185c, a suspected dioxygenase thereby disrupting bacterial homeostasis. Associated with contractive responses of smooth muscles and inhibition of Na+/K+ ATPase. Cytotoxic/Antineoplastic agent. Found to exhibit inhibitory activity against several cancer cell lines, including the drug resistant renal cancer cell line (ACHN). Suppressor of RANKL-induced osteoclastogenesis via down-regulation of c-Fos, NFATc1, NF-kappaB and ERK. Antiprotozoal compound.
Purity:
>97% (HPLC)
SMILES:
C/C(CC[C@H]1C(CC[C@]2([H])[C@]1(C)CCCC2(C)C)=C)=CC[NH]3CN(C)C4=C3C(N)=NC=N4
Solubility Chemicals:
Soluble in DMSO, ethanol or methanol.
Source / Host:
Isolated from sponge Agelas sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Agelasine-A -B, -C and -D, novel bicyclic diterpenoids with a 9-methyladeninium unit possessing inhibitory effects on Na,K-ATPase isolated from the Okinawian sea sponge Agelas sp: H. Nakamura, et al.; Tetrahedron Lett. 25, 2989 (1984) | (+)-agelasine D: improved synthesis and evaluation of antibacterial and cytotoxic activities: A. Vik, et al.; J. Nat. Prod. 69, 381 (2006) | Synthesis, antimicrobial and antineoplastic activities for agelasine and agelasimine analogs with a beta-cyclocitral derived substituent: A. Proszenyak, et al.; Arch. Pharm. 340, 625 (2007) | Antifouling activity of the sponge metabolite agelasine D and synthesised analogs on Balanus improvisus: M. Sjoegren, et al.; Biofouling 24, 251 (2008) | Screening of Agelasine D and Analogs for Inhibitory Activity against Pathogenic Protozoa; Identification of Hits for Visceral Leishmaniasis and Chagas Disease: A. Vik, et al.; Molecules 14, 279 (2009) | From anti-fouling to biofilm inhibition: New cytotoxic secondary metabolites from two Indonesian Agelas sponges: T. Hertiani, et al.; Bioorg. Med. Chem. 18, 1297 (2010) | Synthesis and biological activities of marine terpene-adenine hybrids and synthetic analogs: L.-L. Gundersen; Phytochem. Rev. 12, 467 (2013) (Review) | Identification of the target protein of agelasine D, a marine sponge diterpene alkaloid, as an anti-dormant mycobacterial substance: M. Arai, et al.; Chembiochem. 15, 117 (2014) | Agelasine D suppresses RANKL-induced osteoclastogenesis via down-regulation of c-Fos, NFATc1 and NF-kappaB: M.R. Kang, et al.; Mar. Drugs 12, 5643 (2014)