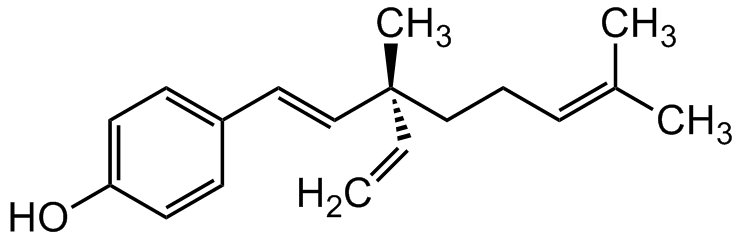
Bakuchiol
Product Code: AG-CN2-0495
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0495-M001 | 1 mg | £40.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
S-Bakuchiol; (+)-Bakuchiol; Bakutrol; Drupanol; Sytenol A; UP 256; (S,E)-4-(3,7-Dimethyl-3-vinylocta-1,6-dien-1-yl)phenol
Appearance:
Yellow to brownish oil.
CAS:
10309-37-2
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light.
InChi:
InChI=1S/C18H24O/c1-5-18(4,13-6-7-15(2)3)14-12-16-8-10-17(19)11-9-16/h5,7-12,14,19H,1,6,13H2,2-4H3/b14-12+/t18-/m1/s1
InChiKey:
LFYJSSARVMHQJB-QIXNEVBVSA-N
Long Description:
Chemical. CAS: 10309-37-2. Formula: C18H24O. MW: 256.4. Isolated from Psoralea corylifolia. Antibiotic. Antimicrobial, anti-inflammatory, anticancer and immunosuppressive compound. Shown to have hepatoprotective and hypoglycemic activity. Apoptosis inducer in cancer cells. Sensitizes cancer cells. Induces cell cycle arrest. Antiviral agent. Inhibited influenza A viral infection and growth through activation of Nrf2. Inhibitor of protein tyrosine phosphatase 1B (PTB1B). Antioxidant. Inhibitor of mitochondrial lipid peroxidation. Inhibitor of inducible nitric oxide synthase (iNOS; NOS II) expression via inactivation of NF-kappaB. DNA polymerase and UDP-glucuronosyltransferase inhibitor. Anti-aging compound with retinol-like effects on gene expression and properties of the skin. Phospholipase A, cyclooxygenase-1 (COX-1) and HIF-1 inhibitor.
MDL:
MFCD01707441
Molecular Formula:
C18H24O
Molecular Weight:
256.4
Package Type:
Vial
Product Description:
Antibiotic. Antimicrobial, anti-inflammatory, anticancer and immunosuppressive compound. Shown to have hepatoprotective and hypoglycemic activity. Apoptosis inducer in cancer cells. Sensitizes cancer cells. Induces cell cycle arrest. Antiviral agent. Inhibited influenza A viral infection and growth through activation of Nrf2. Inhibitor of protein tyrosine phosphatase 1B (PTB1B). Antioxidant. Inhibitor of mitochondrial lipid peroxidation. Inhibitor of inducible nitric oxide synthase (iNOS; NOS II) expression via inactivation of NF-kappaB. DNA polymerase and UDP-glucuronosyltransferase inhibitor. Anti-aging compound with retinol-like effects on gene expression and properties of the skin. Phospholipase A, cyclooxygenase-1 (COX-1) and HIF-1 inhibitor.
Purity:
>98% (HPLC)
SMILES:
OC1=CC=C(/C=C/[C@](C)(C=C)CC/C=C(C)/C)C=C1
Solubility Chemicals:
Soluble in DMSO, ethanol or methanol.
Source / Host:
Isolated from Psoralea corylifolia.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia: N.J. Sun, et al.; J. Nat. Prod. 61, 362 (1998) | Isolation and antihyperglycemic activity of bakuchiol from Otholobium pubescens (Fabaceae), a Peruvian medicinal plant used for the treatment of diabetes: J.M. Krenisky, et al.; Biol. Pharm. Bull. 22, 1137 (1999) | Inhibition of mitochondrial lipid peroxidation by Bakuchiol, a meroterpene from Psoralea corylifolia: H. Haraguchi, et al.; Planta Med. 66, 569 (2000) | In vitro antimicrobial activities of bakuchiol against oral microorganisms: H. Katsura, et al.; Antimicrob. Agents Chemother. 45, 3009 (2001) | Bakuchiol from Psoralea corylifolia inhibits the expression of inducible nitric oxide synthase gene via the inactivation of nuclear transcription factor-kappaB in RAW 264.7 macrophages: H.O. Pae, et al.; Int. Immunopharmacol. 1, 1849 (2001) | Bakuchiol: a hepatoprotective compound of Psoralea corylifolia on tacrine-induced cytotoxicity in Hep G2 cells: H. Cho, et al.; Planta Med. 67, 750 (2001) | Antioxidative components of Psoralea corylifolia (Leguminosae): H. Haraguchi, et al.; Phytother. Res. 16, 539 (2002) | Preparation and in vitro evaluation of radioiodinated bakuchiol as an anti tumor agent: K. Bapat, et al.; Appl. Radiat. Isot. 62, 389 (2005) | In vitro protein tyrosine phosphatase 1B inhibitory phenols from the seeds of Psoralea corylifolia: Y.C. Kim, et al.; Planta Med. 71, 87 (2005) | Bakuchiol-induced caspase-3-dependent apoptosis occurs through c-Jun NH2-terminal kinase-mediated mitochondrial translocation of Bax in rat liver myofibroblasts: E.J. Park, et al.; Eur. J. Pharmacol. 559, 115 (2007) | Bisbakuchiols A and B, novel dimeric meroterpenoids from Psoralea corylifolia: C.-Z. Wu, et al.; Tetrahedr. Lett. 48, 8861 (2007) | Bakuchiol, an antibacterial component of Psoralidium tenuiflorum: P.J. Hsu, et al.; Nat. Prod. Res. 23, 781 (2009) | Isolation and anti-inflammatory activity of Bakuchiol from Ulmus davidiana var. japonica: S.Y. Choi, et al.; J. Med. Food 13, 1019 (2010) | In vitro evidence for bakuchiol's influence towards drug metabolism through inhibition of UDP-glucuronosyltransferase (UGT) 2B7: Y. Xu, et al.; Afr. Health Sci. 14, 564 (2014) | Bakuchiol: A retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects: R.K. Chaudhuri & K. Bojanowski; Int. J. Cosmet. Sci. 36, 221 (2014) | Bakuchiol is a phenolic isoprenoid with novel enantiomer-selective anti-influenza a virus activity involving Nrf2 activation: M. Shoji, et al.; J. Biol. Chem. 290, 28001 (2015) | Bakuchiol sensitizes cancer cells to TRAIL through ROS- and JNK-mediated upregulation of death receptors and downregulation of survival proteins: M.H. Park, et al.; BBRC 473, 586 (2016) | Phytoestrogen Bakuchiol Exhibits In Vitro and In Vivo Anti-breast Cancer Effects by Inducing S Phase Arrest and Apoptosis: L. Li, et al.; Front. Pharmacol. 7,128 (2016) | Bakuchiol suppresses proliferation of skin cancer cells by directly targeting Hck, Blk, and p38 MAP kinase: J.E. Kim, et al.; Oncotarget 7, 14616 (2016) | Identification of UDP-glucuronosyltransferases 1A1, 1A3 and 2B15 as the main contributors to glucuronidation of bakuchiol, a natural biologically active compound: F. Li, et al.; Xenobiotica 47, 369 (2017)