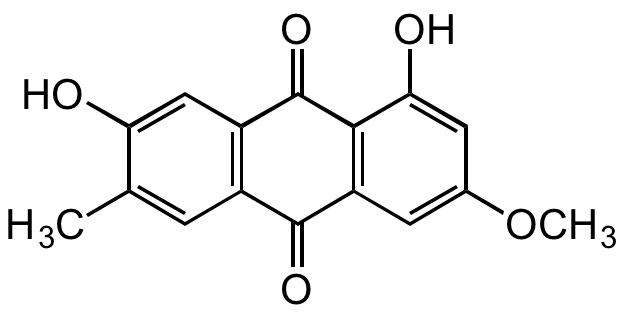
Macrosporin
Product Code: AG-CN2-0497
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0497-M001 | 1 mg | £160.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
1,7-Dihydroxy-3-methoxy-6-methyl-9,10-anthracenedione
Appearance:
Yellow to light orange solid.
CAS:
22225-67-8
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C16H12O5/c1-7-3-9-10(6-12(7)17)16(20)14-11(15(9)19)4-8(21-2)5-13(14)18/h3-6,17-18H,1-2H3
InChiKey:
FKTPLNFTYJEAAB-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 22225-67-8. Formula: C16H12O5. MW: 284.3. Isolated from Stemphylium sp. Anthraquinone antibiotic. Moderate antibacterial agent. Photosensitizer (through ROS production) and leaf necrosis inducer. Antiproliferative. Moderate cytotoxic in selected cancer cells. Shown to inhibit several protein kinases, including Aurora B and FLT3 in low µM range. Mycotoxin. Useful as a reference in food analysis.
MDL:
MFCD14635411
Molecular Formula:
C16H12O5
Molecular Weight:
284.3
Package Type:
Vial
Product Description:
Anthraquinone antibiotic. Moderate antibacterial agent. Photosensitizer (through ROS production) and leaf necrosis inducer. Antiproliferative. Moderate cytotoxic in selected cancer cells. Shown to inhibit several protein kinases, including Aurora B and FLT3 in low µM range. Mycotoxin. Useful as a reference in food analysis.
Purity:
>98% (HPLC)
SMILES:
OC1=CC2=C(C(C(C=C(OC)C=C3O)=C3C2=O)=O)C=C1C
Solubility Chemicals:
Soluble in DMSO, ethanol or methanol.
Source / Host:
Isolated from Stemphylium sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Studies on the metabolic products of Macrosporium porri Elliott Part IV. Structure of Macrosporin (Group III): R. Suemitsu, et al.; Agr. Biol. Chem. 25, 100 (1961) | Biologically active polyketide metabolites from an undetermined fungicolous hyphomycete resembling Cladosporium: U. Hoeller, et al.; J. Nat. Prod. 65, 876 (2002) | Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium: A. Debbab, et al.; J. Nat. Prod. 72, 626 (2009) | Protein kinase inhibitors and other cytotoxic metabolites from the fungal endophyte Stemphylium botryosum isolated from Chenopodium album: A.H. Aly, et al.; Mycosphere - J. Fungal Biol. 1, 153 (2010) | The role of macrosporin in necrotic spots: A. Trigos, et al.; Phytochem. Lett. 4, 122 (2011) | Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. Fungus: C.-J. Zheng, et al.; J. Nat. Prod. 75, 189 (2012)