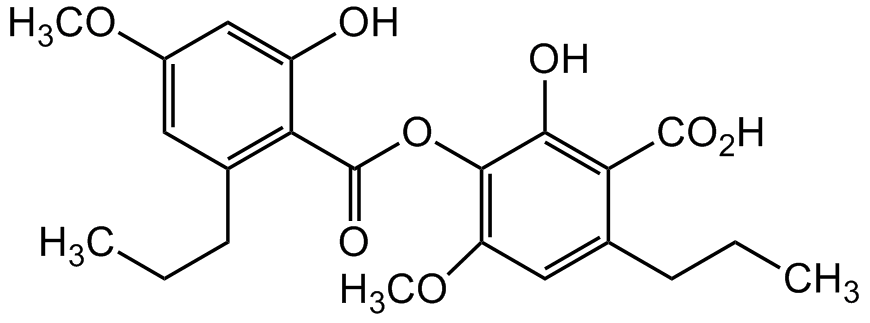
Sekikaic acid
Product Code: AG-CN2-0502
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0502-M001 | 1 mg | £180.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
2-Hydroxy-3-(2-hydroxy-4-methoxy-6-propylbenzoyl)oxy-4-methoxy-6-propylbenzoic acid; Sekicic acid
Appearance:
Solid.
CAS:
607-11-4
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C22H26O8/c1-5-7-12-9-14(28-3)11-15(23)17(12)22(27)30-20-16(29-4)10-13(8-6-2)18(19(20)24)21(25)26/h9-11,23-24H,5-8H2,1-4H3,(H,25,26)
InChiKey:
CPHXGYQLOSNELY-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 607-11-4. Formula: C22H26O8. MW: 418.4. Isolated from Ramalina sp. Antioxidant. Reactive oxygen species (ROS) scavenger. Specific ligand of the coactivator CBP/p300, binding to the GACKIX domain. Efficiently inhibits binding of activators at two binding sites. Antibiotic. Potent antibacterial compound. Antifungal on selected fungi species. Antiviral agent. Interferes with the viral replication of selected respiratory syncytial virus. Competitive alpha-glucosidase and non-competitive beta-glucosidase inhibitor. Shows moderate anticancer activity.
MDL:
N/A
Molecular Formula:
C22H26O8
Molecular Weight:
418.4
Package Type:
Vial
Product Description:
Antioxidant. Reactive oxygen species (ROS) scavenger. Specific ligand of the coactivator CBP/p300, binding to the GACKIX domain. Efficiently inhibits binding of activators at two binding sites. Antibiotic. Potent antibacterial compound. Antifungal on selected fungi species. Antiviral agent. Interferes with the viral replication of selected respiratory syncytial virus. Competitive alpha-glucosidase and non-competitive beta-glucosidase inhibitor. Shows moderate anticancer activity.
Purity:
>96% (HPLC)
SMILES:
OC1=CC(OC)=CC(CCC)=C1C(OC2=C(OC)C=C(CCC)C(C(O)=O)=C2O)=O
Solubility Chemicals:
Soluble in DMSO, ethanol or methanol.
Source / Host:
Isolated from Ramalina sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Phytochemical investigation of the Australian lichens Ramalina glaucescens and Xanthoria parietina: D.A. Dias, et al.; Nat. Prod. Commun. 4, 959 (2009) | Antioxidant activities of edible lichen Ramalina conduplicans and its free radical-scavenging constituents: H. Luo, et al.; Mycosci. 51, 391 (2010) | Antioxidant activity of some lichen metabolites: V.M. Thadhani, et al.; Nat. Prod. Res. 25, 1827 (2011) | Sekikaic acid and lobaric acid target a dynamic interface of the coactivator CBP/p300: C.Y. Majmudar, et al.; Angew. Chem. Int. Ed. Engl. 51, 11258 (2011) | Antimicrobial and toxicological activities of some depsides and depsidones: V.M. Thadhani, et al.; J. Natn. Sci. Found. Sri Lanka 40, 43 (2012) | Glucosidase inhibitory and radical scavenging properties of lichen metabolites Salazinic acid, Sekikaic acid and Usnic acid: L.M. Salazinik, et al.; Hacettepe J. Biol. Chem. 40, 7 (2012) | Antibacterial and antioxidant activity of lichen species Ramalina roesleri: R. Sisodia, et al.; Nat. Prod. Res. 27, 2235 (2013) | Phenolic compounds with in vitro activity against respiratory syncytial virus from the Nigerian lichen Ramalina farinacea: D. Lai, et al.; Planta Med. 79, 1440 (2013) | Chemistry and Biological Activity of Ramalina Lichenized Fungi: A.S. Moreira, et al.; Molecules 20, 8952 (2015) (Review) | Biopharmaceutical potential of two Ramalina lichens and their metabolites: S. Ristic, et al.; Curr. Pharm. Biotechnol. 17, 651 (2016)