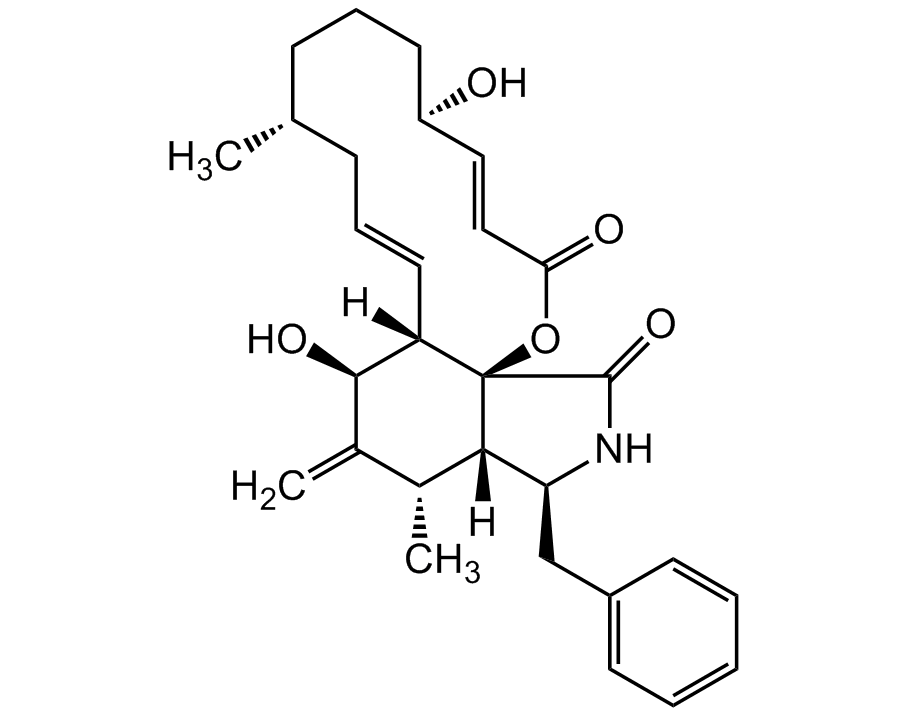
Cytochalasin B
Product Code: AG-CN2-0504
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0504-M001 | 1 mg | £50.00 |
Quantity:
| AG-CN2-0504-M005 | 5 mg | £130.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Phomin; NSC 107658
Appearance:
White solid.
CAS:
14930-96-2
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Keep cool and dry.Protect from light.
Hazards:
H300, H310, H330
InChi:
InChI=1S/C29H37NO5/c1-18-9-7-13-22(31)15-16-25(32)35-29-23(14-8-10-18)27(33)20(3)19(2)26(29)24(30-28(29)34)17-21-11-5-4-6-12-21/h4-6,8,11-12,14-16,18-19,22-24,26-27,31,33H,3,7,9-10,13,17H2,1-2H3,(H,30,34)/b14-8+,16-15+/t18-,19-,22-,23+,24+,26+,27-,29-/m1/s1
InChiKey:
GBOGMAARMMDZGR-TYHYBEHESA-N
Long Description:
Chemical. CAS: 14930-96-2. Formula: C29H37NO5. MW: 479.6. Isolated from Phoma sp. Cell permeable mycotoxin. Actin polymerization inhibitor. Binds to the barbed end of actin, reversibly inhibiting the elongation and shortening of actin filaments. Induces nuclear extrusion. By disrupting actin polymerization, blocks diverse cellular functions, including cell division, migration, phagocytosis, exocytosis and chemotaxis. Induces DNA fragmentation. Used in cytoskeletal reorganization, cell imaging and organelle trafficking studies. Potent antitumor activity. Causes cell cycle arrest and induces apoptosis in cancer cells. Shown to have an inhibitory effect on tumor cell growth without causing immunosuppressive effects. Non-competitive glucose transport inhibitor. Inhibits of GLUT1/GLUT2 mediated monosaccharide transport across the plasma membrane. Does not inhibit GLUT5. Used for testing the genotoxicity of substances in the cytokinesis-block micronucleus cytome (CBMN cyt) assay. Used in cloning through nuclear transfer. Enucleated recipient cells are treated with cytochalasin B. Cytochalasin B makes the cytoplasm of the oocytes more fluid and makes it possible to aspirate the nuclear genome of the oocyte within a small vesicle of plasma membrane into a micro-needle. Decreases the expression levels of DNMT1, DNMT3a, DNMT3b, HAT1 and HDAC1 at the pronuclear stage in porcine embryos. Phosphatidylcholine and phosphatidylethanolamine biosynthesis inhibitor. Platelet aggregation inhibitor.
MDL:
MFCD00077704
Molecular Formula:
C29H37NO5
Molecular Weight:
479.6
Package Type:
Vial
PG:
II
Precautions:
P280, P301, P310, P302, P352, P304, P340, P405
Product Description:
Cell permeable mycotoxin. Actin polymerization inhibitor. Binds to the barbed end of actin, reversibly inhibiting the elongation and shortening of actin filaments. Induces nuclear extrusion. By disrupting actin polymerization, blocks diverse cellular functions, including cell division, migration, phagocytosis, exocytosis and chemotaxis. Induces DNA fragmentation. Used in cytoskeletal reorganization, cell imaging and organelle trafficking studies. Potent antitumor activity. Causes cell cycle arrest and induces apoptosis in cancer cells. Shown to have an inhibitory effect on tumor cell growth without causing immunosuppressive effects. Non-competitive glucose transport inhibitor. Inhibits of GLUT1/GLUT2 mediated monosaccharide transport across the plasma membrane. Does not inhibit GLUT5. Used for testing the genotoxicity of substances in the cytokinesis-block micronucleus cytome (CBMN cyt) assay. Used in cloning through nuclear transfer. Enucleated recipient cells are treated with cytochalasin B. Cytochalasin B makes the cytoplasm of the oocytes more fluid and makes it possible to aspirate the nuclear genome of the oocyte within a small vesicle of plasma membrane into a micro-needle. Decreases the expression levels of DNMT1, DNMT3a, DNMT3b, HAT1 and HDAC1 at the pronuclear stage in porcine embryos. Phosphatidylcholine and phosphatidylethanolamine biosynthesis inhibitor. Platelet aggregation inhibitor.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
C[C@@H]1C([C@@H](O)[C@@]2([H])[C@@]3(OC(/C=C/[C@H](O)CCC[C@@H](C)C/C=C/2)=O)[C@]1([H])[C@H](CC4=CC=CC=C4)NC3=O)=C
Solubility Chemicals:
Soluble in DMSO, methanol, ethanol or DMF.
Source / Host:
Isolated from Drechslera dematoidea.
Transportation:
Excepted Quantity
UN Nummer:
UN 1544
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Cytochalasin B. 3. Inhibition of human polymorphonuclear leukocyte phagocytosis: A.T. Davis, et al.; Proc. Soc. Exp. Biol. Med. 137, 161 (1971) | Cytochalasin B: Inhibition of glucose and glucosamine transport: R.D. Estensen & P.G.W. Plagemann; PNAS 69, 1430 (1972) | Enucleation of mammalian cells with cytochalasin B: D.M. Prescott, et al.; Exp. Cell Res. 71, 480 (1972) | Inhibition and reversal of platelet activation by cytochalasin B or colcemid: M.M. Boyle Kay & H.H. Fudenberg; Nature 244, 288 (1973) | Cytochalasin B: inhibition of glucose-induced insulin release from isolated rat pancreatic islets: P. Schauders & H. Frerichs; Diabetologia 10, 85 (1974) | Reversible inhibition of the motility of human spermatozoa by cytochalasin B: R.N. Peterson & M. Freund; Fertil. Steril. 28, 257 (1977) | Effects of cytochalasin B on endocytosis and exocytosis: P. Davies & A.C. Allison; Front. Biol. 46, 143 (1978) | Mechanism of action of cytochalasin B on actin: S: MacLean-Fletcher & T.D. Pollard; Cell. 20, 329 (1980) | Effect of cytochalasin B on glucose uptake, utilization, oxidation and insulinotropic action in tumoral insulin-producing cells: W.J. Malaisse; Cell. Biochem. Funct. 5, 183 (1987) | Cytochalasin B induces cellular DNA fragmentation: M.A. Kolber, et al.; FASEB J. 4, 3021 (1990) | Inhibition of Phosphatidylcholine and Phosphatidylethanoamine Biosynthesis by Cytochalasin B in Cultured Glioma Cells: Potential Regulation of Biosynthesis by Ca2+-Dependent Mechanisms: T.P. George, et al.; Biochim. Biophys. Acta 1084, 185 (1991) | Cytochalasin B may shorten actin filaments by a mechanism independent of barbed end capping: P.A. Theodoropoulos, et al.; Biochem. Pharmacol. 47, 1875 (1994) | Production of mice entirely derived from embryonic stem (ES) cell with many passages by coculture of ES cells with cytochalasin B induced tetraploid embryos: O. Ueda; Exp. Anim. 44, 205 (1995) | The inhibition of GLUT1 glucose transport and cytochalasin B binding activity by tricyclic antidepressants: H.B. Pinkofsky, et al.; Life. Sci. 66, 271 (2000) | Cytokinesis-block micronucleus cytome assay: M. Fenech; Nat. Protocols 2, 1084 (2007) | The chemistry and biology of cytochalasans: K. Scherlach, et al.; Nat. Prod. Rep. 27, 869 (2010) (Review) | Cytochalasin B treatment of mouse oocytes during intracytoplasmic sperm injection (ICSI) increases embryo survival without impairment of development: L.L. Hu, et al.; Zygote 20, 361 (2012) | Cytochalasin B induces apoptosis through the mitochondrial apoptotic pathway in HeLa human cervical carcinoma cells: J. Hwang, et al.; Oncol. Rep. 30, 1929 (2013) | Preparation, In Vivo Administration, Dose-Limiting Toxicities, and Antineoplastic Activity of Cytochalasin B: M. Trendowski, et al.; Transl. Oncol. 8, 308 (2015) | Mechanisms underlying effect of the mycotoxin cytochalasin B on induction of cytotoxicity, modulation of cell cycle, Ca2+ homeostasis and ROS production in human breast cells: H.T. Chang, et al.; Toxicol. 31, 1 (2016) | Effects of cytochalasin B on DNA methylation and histone modification in parthenogenetically activated porcine embryos: X. Hou, et al.; Reproduction 152, 519 (2016)