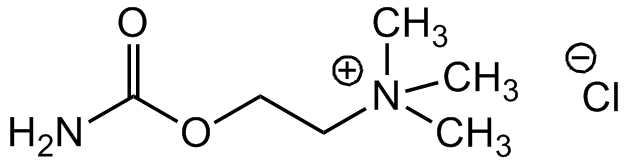
Carbamoylcholine chloride
Product Code: AG-CR1-3649
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CR1-3649-M100 | 100 mg | £45.00 |
Quantity:
| AG-CR1-3649-G001 | 1 g | £80.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Carbachol; (2-Hydroxyethyl)trimethylammonium chloride carbamate
Appearance:
White to yellow crystalline solid.
CAS:
51-83-2
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Hygroscopic.Keep cool and dry.
Hazards:
H300
InChi:
InChI=1S/C6H14N2O2.ClH/c1-8(2,3)4-5-10-6(7)9;/h4-5H2,1-3H3,(H-,7,9);1H
InChiKey:
AIXAANGOTKPUOY-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 51-83-2. Formula: C6H15N2O2 . Cl. MW: 182.7. Acetylcholine analog that activates acetylcholine receptors (AChR). Non-selective agonist of nicotinic (nAChR) and muscarinic (mAChR) receptors that is resistant to the action of cholinesterases. Used to study responses mediated by nAChR and mAChR, including smooth muscle contraction, gut motility and neuronal signaling. Shown to inhibit apoptotic death of cultured neurons.
MDL:
MFCD00012011
Molecular Formula:
C6H15N2O2 . Cl
Molecular Weight:
147.3 . 35.4
Package Type:
Plastic Vial
PG:
II
Precautions:
P301, P310
Product Description:
Acetylcholine analog that activates acetylcholine receptors (AChR). Non-selective agonist of nicotinic (nAChR) and muscarinic (mAChR) receptors that is resistant to the action of cholinesterases. Used to study responses mediated by nAChR and mAChR, including smooth muscle contraction, gut motility and neuronal signaling. Shown to inhibit apoptotic death of cultured neurons.
Purity:
>98%
Signal word:
Danger
SMILES:
NC(OCC[N+](C)(C)C)=O.[Cl-]
Solubility Chemicals:
Soluble in water, methanol, ethanol or DMSO.
Transportation:
Excepted Quantity
UN Nummer:
UN 2811
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Activation of muscarinic cholinergic receptors blocks apoptosis of cultured cerebellar granule neurons: G.M. Yan, et al.; Mol. Pharmacol. 47, 248 (1995) | Carbamoylcholine homologs: Novel and potent agonists at neuronal nicotinic acetylcholine receptors: A.A. Jensen, et al.; Mol. Pharmacol. 64, 865 (2003) | The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells: Y. Xiao & K.J. Kellar; J. Pharmacol. Exp. Ther. 310, 98 (2004) | Distinct pathways of ERK activation by the muscarinic agonists pilocarpine and carbachol in a human salivary cell line: A.L. Lin, et al.; Am. J. Physiol. Cell Physiol. 294, C1454 (2008) | K+ACh channel activation with carbachol increases atrial ANP release: D.Y. Xu, et al.; Life Sci. 82, 1083 (2008) | IL-17A induces hypo-contraction of intestinal smooth muscle via induction of iNOS in muscularis macrophages: D. Mori, et al.; J. Pharmacol. Sci. 125, 394 (2014)