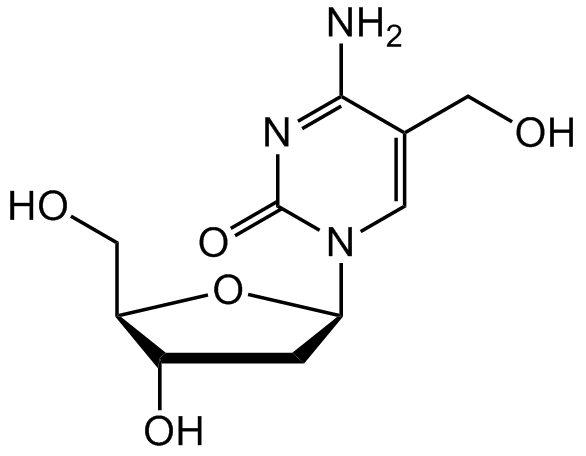
5-(Hydroxymethyl)-2'-deoxycytidine
| Code | Size | Price |
|---|
| AG-CR1-3532-M001 | 1 mg | £40.00 |
Quantity:
| AG-CR1-3532-M005 | 5 mg | £120.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
5 hmdC; 2'-Deoxy-5-(hydroxymethyl)-cytidine
Appearance:
White solid.
CAS:
7226-77-9
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Hygroscopic.Keep cool and dry.Protect from moisture.
InChi:
InChI=1S/C10H15N3O5/c11-9-5(3-14)2-13(10(17)12-9)8-1-6(16)7(4-15)18-8/h2,6-8,14-16H,1,3-4H2,(H2,11,12,17)/t6-,7+,8+/m0/s1
InChiKey:
HMUOMFLFUUHUPE-XLPZGREQSA-N
Long Description:
Chemical. CAS: 7226-77-9. Formula: C10H15N3O5. MW: 257.2. Synthetic. Epigenetic base. A modified pyrimidine that is capable of producing interstrand cross-links in double-stranded DNA and has been used to quantify DNA hydroxymethylation levels in biological samples. Enriched in the brain, suggesting a role in epigenetic control of neuronal function. Used in epigenetic research and important for cancer research. Potent HIV-1 reverse transcriptase activity inhibitor. Can be used as building block in nucleoside and nucleotide chemistry.
MDL:
MFCD09842113
Molecular Formula:
C10H15N3O5
Molecular Weight:
257.2
Package Type:
Vial
Product Description:
Epigenetic base. A modified pyrimidine that is capable of producing interstrand cross-links in double-stranded DNA and has been used to quantify DNA hydroxymethylation levels in biological samples. Enriched in the brain, suggesting a role in epigenetic control of neuronal function. Used in epigenetic research and important for cancer research. Potent HIV-1 reverse transcriptase activity inhibitor. Can be used as building block in nucleoside and nucleotide chemistry.
Purity:
>98%
SMILES:
O[C@H]1C[C@H](N2C=C(CO)C(N)=NC2=O)O[C@@H]1CO
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or water (5mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Interstrand cross-link formation in duplex and triplex DNA by modified pyrimidines: X. Peng, et al.; JACS 130, 10299 (2008) | The nuclear DNA base, 5-hydroxymethylcytosine is present in brain and enriched in Purkinje neurons: S. Kriaucionis & N. Heintz; Science 324, 929 (2009) | 5-Modified-2'-dU and 2'-dC as mutagenic anti HIV-1 proliferation agents: synthesis and activity: Y. El Sadafi, et al.; J. Med. Chem. 53, 1534 (2010) | Determination of genomic 5-hydroxymethyl-2'-deoxycytidine in human DNA by capillary electrophoresis with laser induced fluorescence: A.M. Krais, et al.; Epigenetics 6, 560 (2011) | Detection of oxidation products of 5-methyl-2'-deoxycytidine in Arabidopsis DNA: S. Liu, et al.; PLoS One 8, e84620 (2013) | Synthesis of a DNA promoter segment containing all four epigenetic nucleosides: 5-Methyl-, 5-hydroxymethyl-, 5-formyl-, and 5-carboxy-2'-deoxycytidine: A.S. Schroeder, et al.; Angew. Chem. Int. Ed. 53, 315 (2014) | Hydroxyl-radical-induced oxidation of 5-methylcytosine in isolated and cellular DNA: G.S. Madugundu, et al.; Nucleic Acids Res. 42, 7450 (2014) | CDA directs metabolism of epigenetic nucleosides revealing a therapeutic window in cancer; M. Zauri, et al.; Nature 524, 114 (2015)
Related Products
| Product Name | Product Code | Supplier | 2'-Deoxy-5-formylcytidine | AG-CR1-3531 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|