Bafilomycin D (high purity)
| Code | Size | Price |
|---|
| BVT-0475-M001 | 1 mg | £205.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Tubamycin; Antibiotic 3D5
Appearance:
Off-white to yellow solid.
CAS:
98813-13-9
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light when in solution.
Hazards:
H302, H312, H332
InChi:
InChI=1S/C35H56O8/c1-20(2)31(37)23(5)15-16-28(36)26(8)33(39)27(9)34-29(41-10)14-12-13-21(3)17-24(6)32(38)25(7)18-22(4)19-30(42-11)35(40)43-34/h12-16,18-20,23-27,29,31-34,37-39H,17H2,1-11H3/b14-12+,16-15+,21-13+,22-18+,30-19+/t23-,24-,25+,26+,27-,29-,31+,32-,33-,34+/m0/s1
InChiKey:
ZKOTUWJMGBWBEO-YXTSRGFTSA-N
Long Description:
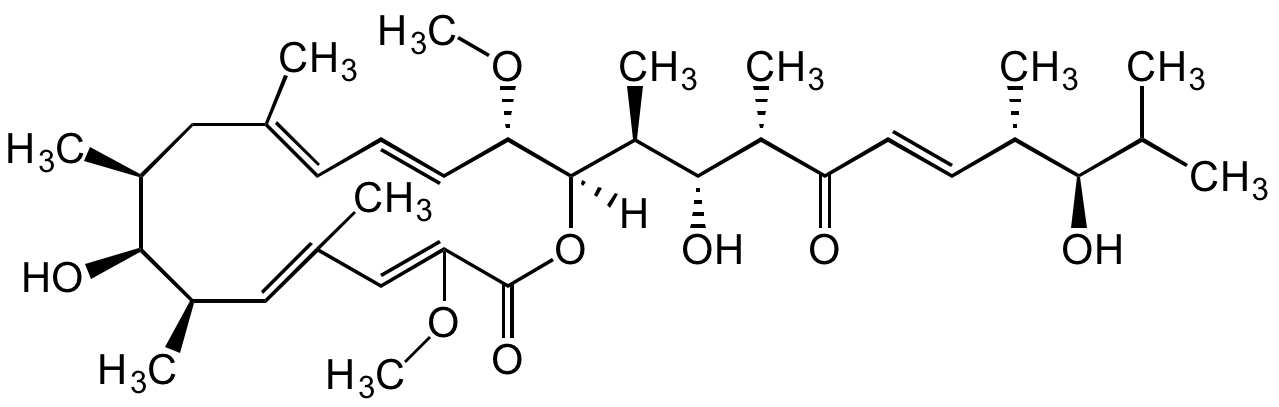
Chemical. CAS: 98813-13-9. Formula: C35H56O8. MW: 604.8. Semi-synthetic product formed from Bafilomycin B1. Macrolide antibiotic. Bafilomycin derivative, in which the characteristic hemiketal portion has been opened. Potent and specific vacuolar-type H+-ATPase inhibitor (V-ATPase). Antiproliferative agent against selected human glioma cell lines. Cytoxic activity. Active against Gram-positive bacteria and plant pathogen fungi. Shows weak insecticidal and anti-nematodic activity.
MDL:
MFCD14635429
Molecular Formula:
C35H56O8
Molecular Weight:
604.8
Package Type:
Plastic Vial
Precautions:
P261, P301, P312, P302, P352, P304, P340
Product Description:
Macrolide antibiotic. Bafilomycin derivative, in which the characteristic hemiketal portion has been opened. Potent and specific vacuolar-type H+-ATPase inhibitor (V-ATPase). Antiproliferative agent against selected human glioma cell lines. Cytoxic activity. Active against Gram-positive bacteria and plant pathogen fungi. Shows weak insecticidal and anti-nematodic activity.
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
CO/C1=C/C(C)=C/[C@@H](C)[C@@H](O)[C@@H](C)C/C(C)=C/C=C/[C@H](OC)[C@]([C@@H](C)[C@@H](O)[C@H](C)C(/C=C/[C@H](C)[C@H](O)C(C)C)=O)([H])OC1=O
Solubility Chemicals:
Soluble in DMSO, 100% ethanol, methanol, acetone or dichloromethane.
Source / Host:
Semi-synthetic product formed from Bafilomycin B1.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
The structures of novel insecticidal macrolides: bafilomycins D and E, and oxohygrolidin: A. Kretschmer, et al.; Agric. Biol. Chem. 49, 2509 (1985) | Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases: S. Droese, et al.; Biochem. 32, 3902 (1993) | Bafilolides, potent inhibitors of the motility and development of the free-living stages of parasitic nematodes: E. Lacey, et al.; Int. J. Parasitol. 25, 349 (1995) | Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases: S. Droese & K. Altendorf; J. Exp. Biol. 200, 1 (1997) | Absolute configurations of macrolide antibiotics of the bafilomycin and leucanicidin groups: M. G. O'Shea, et al.; J. Antibiot. 50, 1073 (1997) | Bafilomycins and odoriferous sesquiterpenoids from Streptomyces albolongus isolated from Elephas maximus feces: N. Ding, et al.; J. Nat. Prod. 79, 799 (2016) | Bioactive bafilomycins and a new N -arylpyrazinone derivative from marine-derived Streptomyces sp. HZP-2216E: Z. Zhang, et al.; Planta Med. 83, 1405 (2017)