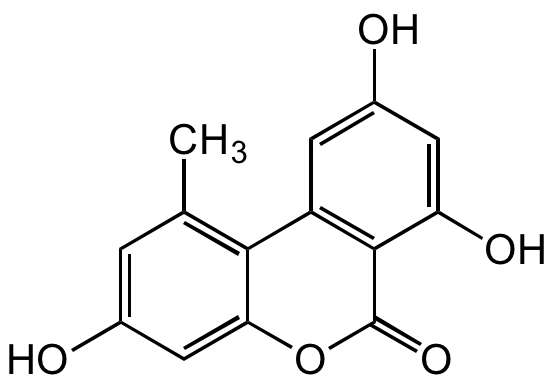
Alternariol
| Code | Size | Price |
|---|
| BVT-0465-M001 | 1 mg | £85.00 |
Quantity:
| BVT-0465-M005 | 5 mg | £255.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+40
Images
Documents
Further Information
Alternate Names/Synonyms:
AOH; NSC 638263; 3,4',5-Trihydroxy-6'-methyldibenzo-alpha-pyrone
Appearance:
Light brown solid.
CAS:
641-38-3
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Keep cool and dry.
Hazards:
H300, H310, H330, H360
InChi:
InChI=1S/C14H10O5/c1-6-2-7(15)5-11-12(6)9-3-8(16)4-10(17)13(9)14(18)19-11/h2-5,15-17H,1H3
InChiKey:
CEBXXEKPIIDJHL-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 641-38-3. Formula: C14H10O5. MW: 258.2. Isolated from Alternaria sp. Mycotoxin. Phytotoxin. Shows cytotoxic, teratogenic, mutagenic and genotoxic properties by DNA intercalation. Induces several types of DNA damage, including oxidative DNA damage, single-stranded DNA breaks (SSB) and double-strand, through AOH-induced ROS production and interaction with DNA topoisomerase. Inhibits the activity of topoisomerase IIalpha in mammalian cells. Induces cell growth arrest in G2/M phase. GSK-3beta inhibitor (sub-µM IC50 values). Antiviral activity (herpes simplex). Hepatitis C NS3-4A protease inhibitor. Induces cytochrome P450 1A1 expression and apoptosis in mouse hepatoma cells.
MDL:
MFCD00133068
Molecular Formula:
C14H10O5
Molecular Weight:
258.2
Package Type:
Plastic Vial
PG:
III
Precautions:
P201, P260, P262, P280, P284, P301, P310, P302, P350, P310, P405
Product Description:
Mycotoxin. Phytotoxin. Shows cytotoxic, teratogenic, mutagenic and genotoxic properties by DNA intercalation. Induces several types of DNA damage, including oxidative DNA damage, single-stranded DNA breaks (SSB) and double-strand, through AOH-induced ROS production and interaction with DNA topoisomerase. Inhibits the activity of topoisomerase IIalpha in mammalian cells. Induces cell growth arrest in G2/M phase. GSK-3beta inhibitor (sub-µM IC50 values). Antiviral activity (herpes simplex). Hepatitis C NS3-4A protease inhibitor. Induces cytochrome P450 1A1 expression and apoptosis in mouse hepatoma cells.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
OC1=CC(C2=C(C)C=C(O)C=C2O3)=C(C(O)=C1)C3=O
Solubility Chemicals:
Soluble in DMSO (20mg/ml) or DMF. Slightly soluble in acetone (1mg/ml).
Source / Host:
Isolated from Alternaria sp.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at +4°C. After reconstitution protect from light at -20°C.
References
Studies in the biochemistry of micro-organisms. 90. Alternariol and alternariol monomethyl ether, metabolic products of Alternariatenuis: H. Raistrick, et al.; Biochem. J. 55, 421 (1953) | Alternariol, a dibenzopyrone mycotoxin of Alternaria spp. is a new photosensitizing and DNA cross-linking agent: F. DiCosmo & N.A. Straus; Experientia 41, 1188 (1985) | Evaluation of alternariol and alternariol methyl ether for mutagenic activity in Salmonella typhimurium: V.M. Davis & M.E. Stack; Appl. Environ. Microbiol. 60, 3901 (1994) | Alternariol acts as a topoisomerase poison preferentially affecting the IIalpha isoform.: M. Fehr, et al.; Mol. Nutr. Food Res. 53, 441 (2009) | Mechanism of Alternariol monomethyl ether-induced mitochondrial apoptosis in human colon carcinoma cells: F. Bensassi, et al.; Toxicology 290, 230 (2011) | Heptaketides with antiviral activity from three endolichenic fungal strains Nigrospora sp. Alternaria sp. and Phialophora sp.: J.-W. He, et al.; Fitoterapia 83, 1087 (2012) | Alternariol: D. Siegel, et al.; Acta Crystallogr. Sec. E Struct. Rep. Online 66, o1366 (2010) | The Fungal Phytotoxin Alternariol 9-Methyl Ether and Some of Its Synthetic Analogues Inhibit the Photosynthetic Electron Transport Chain: A. Demuner, et al.; J. Nat. Prod. 76, 2234 (2013) | Modulation of the cellular redox status by the Alternaria toxins alternariol and alternariol monomethyl ether: C. Tiessen, et al.; Toxicol. Lett. 216, 23 (2013) | Mycotoxins from Alternaria: toxicological implications: C. Dall'Asta, et al.; Adv. Mol. Toxicol. 8, 107 (2014) | Impact of Alternaria toxins on CYP1A1 expression in different human tumor cells and relevance for genotoxicity: G. Pahlke, et al.; Toxicol. Lett. 240, 93 (2016) | Marine fungi as producers of benzocoumarins, a new class of inhibitors of glycogen-synthase-kinase 3beta: J. Wiese, et al.; Mar. Drugs 2016, 14 (2016) | Involvement of p38MAPK-ATF2 signaling pathway in alternariol induced DNA polymerase beta expression: Z. Jimin, et al.; Oncol. Lett. 12, 675 (2016) | Alternariol induce toxicity via cell death and mitochondrial damage on Caco-2 cells: C. Fernandez-Blanco, et al.; Food Chem. Toxicol. 88, 32 (2016) | Mechanisms of Action and Toxicity of the Mycotoxin Alternariol: A Review: A. Solhaug, et al.; Basic Clin. Pharmacol. Toxicol. 119, 533 (2016) | The Alternaria alternata Mycotoxin alternariol suppresses lipopolysaccharide-induced inflammation: S. Grover & C. B. Lawrence; Int. J. Mol. Sci. 2017, 18 (2017) | Impact of phase I metabolism on uptake, oxidative stress and genotoxicity of the emerging mycotoxin alternariol and its monomethyl ether in esophageal cells: C. Tiessen, et al.; Arch. Toxicol. 91, 1213 (2017) | Dual effectiveness of alternaria but not fusarium mycotoxins against human topoisomerase II and bacterial gyrase: K. Jarolim, et al.; Arch. Toxicol. 91, 2007 (2017) | Genistein and delphinidin antagonize the genotoxic effects of the mycotoxin alternariol in human colon carcinoma cells: G. Aichinger, et al.; Mol. Nutr. Food Res. 2017, 61 (2017) | Nonsteroidal mycotoxin alternariol is a full androgen agonist in the yeast reporter androgen bioassay: S. Stypula-Trebas, et al.; Environ. Toxicol. Pharmacol. 55, 208 (2017)
Related Products
| Product Name | Product Code | Supplier | Terrein | BVT-0193 | Bioviotica | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agistatin E | BVT-0231 | Bioviotica | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||