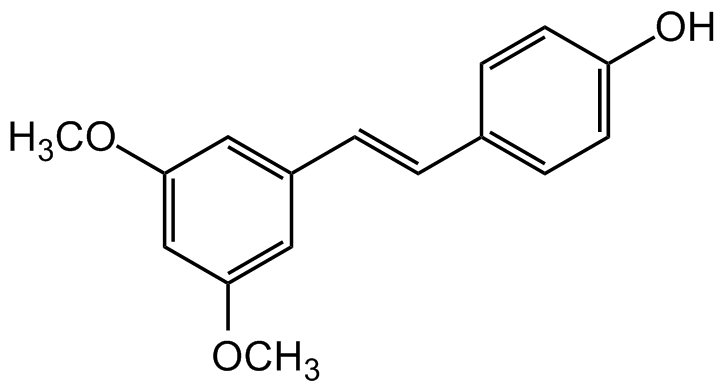
Pterostilbene
| Code | Size | Price |
|---|
| CDX-P0234-M010 | 10 mg | £72.00 |
Quantity:
| CDX-P0234-M100 | 100 mg | £145.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Images
Documents
Further Information
Alternate Names/Synonyms:
trans-3,5-Dimethoxy-4'-Hydroxystilbene; 4-[(1E)-2-(3,5-Dimethoxyphenyl)ethenyl]phenol; 3',5'-Dimethoxy-4-Stilbenol
Appearance:
Off-white to yellow powder.
CAS:
537-42-8
Class:
9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS05,GHS09
Handling Advice:
Protect from light and moisture.
Hazards:
H318, H411
InChi:
InChI=1S/C16H16O3/c1-18-15-9-13(10-16(11-15)19-2)4-3-12-5-7-14(17)8-6-12/h3-11,17H,1-2H3/b4-3+
InChiKey:
VLEUZFDZJKSGMX-ONEGZZNKSA-N
Long Description:
Chemical. CAS: 537-42-8. Formula: C16H16O3. MW: 256.3. Synthetic. Cell permeable natural methoxylated analog of resveratrol. Antioxidant, antiproliferative, anti-inflammatory, anti-hyperglycemic and anti-diabetic agent. Induces apoptosis. Induces Autophagy. Pterostilbene is a naturally-occurring dimethyl ether analog of resveratrol that is abundant in blueberries. Like resveratrol, pterostilbene acts as a powerful antioxidant, suppresses the synthesis of prostaglandin E2 from lipopolysaccharide-stimulated human peripheral blood mononuclear cells (IC50 = 1.0 µM for pterostilbene, 3.2 µM for resveratrol), and inhibits cell proliferation (IC50 = ~60 µM for both compounds). Very potent inhibitor of cytochrome P450 1A1 (CYP1A1) with Ki=0.57µM. Pterostilbene blocks the activation of ERK1/2, p38 MAPK, and PI3K/Akt signaling pathways, reducing NF-kappaB and AP-1 transcriptional activation. Moderately inhibits COX-1 and COX-2 (IC50=19.8µM and 83.9µM).
MDL:
MFCD00238710
Molecular Formula:
C16H16O3
Molecular Weight:
256.3
Package Type:
Vial
PG:
III
Precautions:
P273, P280, P305+P351+P338
Product Description:
Cell permeable natural methoxylated analog of resveratrol. Antioxidant, antiproliferative, anti-inflammatory, anti-hyperglycemic and anti-diabetic agent. Induces apoptosis. Induces Autophagy. Pterostilbene is a naturally-occurring dimethyl ether analog of resveratrol that is abundant in blueberries. Like resveratrol, pterostilbene acts as a powerful antioxidant, suppresses the synthesis of prostaglandin E2 from lipopolysaccharide-stimulated human peripheral blood mononuclear cells (IC50 = 1.0 µM for pterostilbene, 3.2 µM for resveratrol), and inhibits cell proliferation (IC50 = ~60 µM for both compounds). Very potent inhibitor of cytochrome P450 1A1 (CYP1A1) with Ki=0.57µM. Pterostilbene blocks the activation of ERK1/2, p38 MAPK, and PI3K/Akt signaling pathways, reducing NF-kappaB and AP-1 transcriptional activation. Moderately inhibits COX-1 and COX-2 (IC50=19.8µM and 83.9µM).
Purity:
>97% (HPLC)
Signal word:
Danger
SMILES:
OC(C=C1)=CC=C1/C=C/C2=CC(OC)=CC(OC)=C2
Solubility Chemicals:
Soluble in DMSO (20 mg/ml), methanol (10 mg/ml), DMF (30 mg/ml) or ethanol (30 mg/ml). Insoluble in water.
Source / Host:
Synthetic.
Transportation:
Excepted Quantity
UN Nummer:
UN3077
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) M. Manickam, et al.; J. Nat. Prod. 60, 609 (1997) | (2) A.M. Rimando, et al.; J. Agric. Food Chem. 50, 3453 (2002) | (3) M. Roberti, et al.; J. Med. Chem. 46, 3546 (2003) | (4) R. Amorati, et al.; J. Org. Chem. 69, 7101 (2004) | (5) S. Hougee, et al.; Planta Med. 71, 387 (2005) | (6) P. Ferrer, et al.; Neoplasia 7, 37 (2005) | (7) A.M. Rimando, et al.; J. Agric. Food Chem. 53, 3403 (2005) | (8) R. Mikstacka, et al.; Xenobiotica 36, 269 (2006) | (9) K.A. Roupe, et al.; Curr. Clin. Pharmacol. 1, 81 (2006) | (10) R. Mikstacka, et al.; Mol. Nutr. Food Res. 51, 517 (2007) | (11) M.H. Pan, et al.; J. Agric. Food Chem. 55, 7777 (2007) | (12) B. Billack, et al.; Cell. Mol. Biol. Lett. 13, 553 (2008) | (13) C.M. Remsberg, et al.; Phytother. Res. 22, 169 (2008) | (14) M. Cichocki, et al.; Mol. Nutr. Food Res. 52, S62 (2008) | (15) M.H. Pan, et al.; Carcinogenesis 30, 1234 (2009)
Related Products
| Product Name | Product Code | Supplier | Oxyresveratrol | CDX-O0035 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resveratrol | CDX-R0057 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||