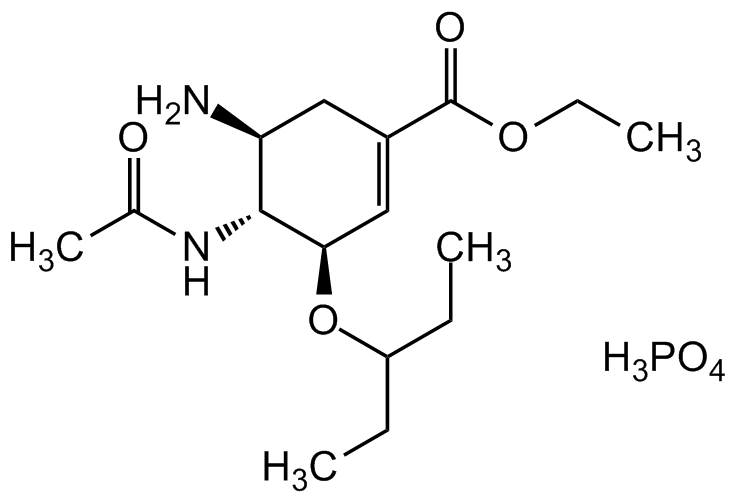
Oseltamivir . phosphate
| Code | Size | Price |
|---|
| AG-CR1-3714-M025 | 25 mg | £35.00 |
Quantity:
| AG-CR1-3714-M100 | 100 mg | £50.00 |
Quantity:
| AG-CR1-3714-M250 | 250 mg | £105.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
GS-4104; Ro 64-0796/002; Tamiflu
Appearance:
White to off-white solid.
CAS:
204255-11-8
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H317 , H319, H412
InChi:
InChI=1S/C16H28N2O4.HO4P.H2/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19;1-4-5(2)3;/h9,12-15H,5-8,17H2,1-4H3,(H,18,19);1H;1H/t13-,14+,15+;;/m0./s1
InChiKey:
POPJIXMLXGJJOV-ZFSDTUNKSA-N
Long Description:
Chemical. CAS: 204255-11-8. Formula: C16H28N2O4 . H3PO4. MW: 312.4 . 98.0. Oseltamivir phosphate is an influenza viral neuraminidase inhibitor. It is an antiviral prodrug targeted against the influenza viruses. Once hydrolyzed in the liver to its active metabolite, oseltamivir carboxylate, it can competitively inhibit viral neuraminidase (IC50s=0.1-4.9nM for influenza neuraminidases A and B). The enzyme cleaves the sialic acid which is found on glycoproteins on the surface of human cells that helps new virions to exit the cell. Thus oseltamivir is blocking the release of new viral particles from a host cell. Oseltamivir phosphate is used clinically to treat influenza A and influenza B, and to prevent flu after exposure. It potentially could be used to reduce the spread of the SARS-CoV2 and counteract against the transmission of COVID-19.
MDL:
MFCD08059548
Molecular Formula:
C16H28N2O4 . H3PO4
Molecular Weight:
312.4 . 98.0
Package Type:
Vial
Precautions:
P280, P302+P352, P333+P313, P305+P351+P338, P273
Product Description:
Oseltamivir phosphate is an influenza viral neuraminidase inhibitor. It is an antiviral prodrug targeted against the influenza viruses. Once hydrolyzed in the liver to its active metabolite, oseltamivir carboxylate, it can competitively inhibit viral neuraminidase (IC50s=0.1-4.9nM for influenza neuraminidases A and B). The enzyme cleaves the sialic acid which is found on glycoproteins on the surface of human cells that helps new virions to exit the cell. Thus oseltamivir is blocking the release of new viral particles from a host cell. Oseltamivir phosphate is used clinically to treat influenza A and influenza B and to prevent flu after exposure. It potentially could be used to reduce the spread of the SARS-CoV-2 and counteract against the transmission of COVID-19.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
O=P(OO)=O.N[C@@H]1[C@@H](NC(C)=O)[C@H](OC(CC)CC)C=C(C(OCC)=O)C1.[HH]
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or water (10mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor: R.W. Sidwell, et al.; Antiviral Res. 37, 107 (1998) | Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza: F.G. Hayden, et al.; N. Engl. J. Med. 341, 1336 (1999) | Identification of GS 4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS 4071: W. Li, et al.; Antimicr. Agents Chemother. 42, 647 (1998) | Biexponential decomposition of a neuraminidase inhibitor prodrug (GS-4104) in aqueous solution: R. Oliyai, et al.; Pharm. Res. 15, 1300 (1998) | Carbocyclic influenza neuraminidase inhibitors possessing a C3-cyclic amine side chain: Synthesis and inhibitory activity: W. Lew, et al.; Bioorg. Med. Chem. Lett. 10, 1257 (2000) | Discovery and development of GS 4104 (oseltamivir): an orally active influenza neuraminidase inhibitor: W. Lew, et al.; Curr. Med. Chem. 7, 663 (2000) (Review) | Synthesis of potent pyrrolidine influenza neuraminidase inhibitors: A.C. Krueger, et al.; Bioorg. Med. Chem. Lett. 18, 1692 (2008) | Susceptibility of highly pathogenic H5N1 influenza viruses to the neuraminidase inhibitor oseltamivir differs in vitro and in a mouse model: E.A. Govorkova, et al.; Antimicr. Agents Chemother. 53, 3088 (2009) | Coronavirus puts drug repurposing on the fast track: C. Harrison; Nat. Biotech. NEWS (2020)