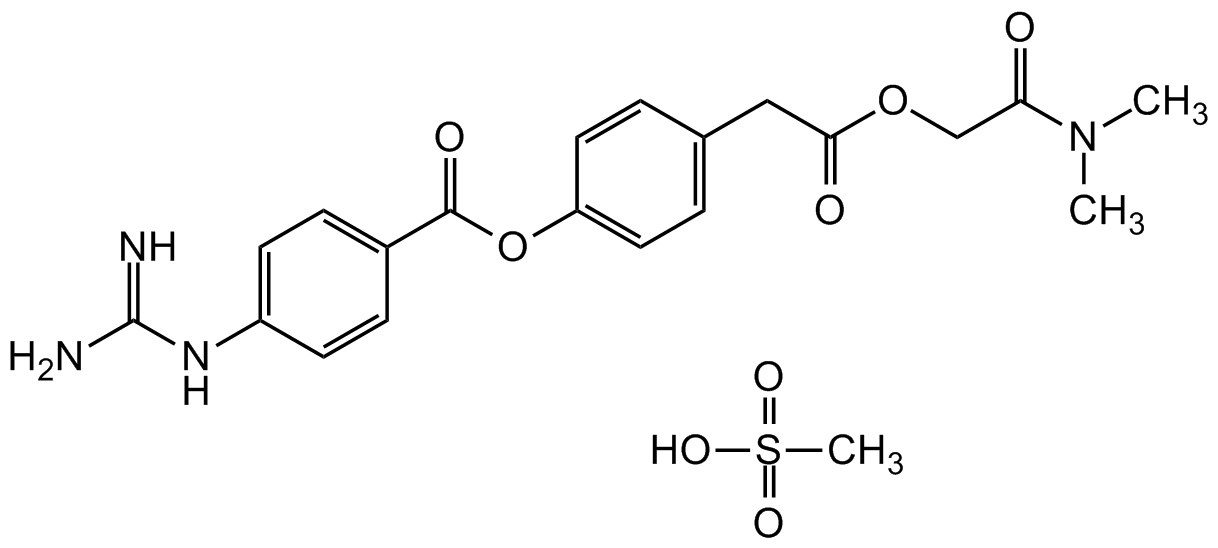
Camostat . mesylate
| Code | Size | Price |
|---|
| AG-CR1-3716-M010 | 10 mg | £40.00 |
Quantity:
| AG-CR1-3716-M050 | 50 mg | £130.00 |
Quantity:
| AG-CR1-3716-M250 | 250 mg | £400.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
FOY 305; Foipan; FOY-S 980; Camostat mesilate
Appearance:
White to off-white solid.
CAS:
59721-29-8
Class:
9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS09
Hazards:
H302, H312, H319, H332, H410
InChi:
InChI=1S/C20H22N4O5.CH4O3S/c1-24(2)17(25)12-28-18(26)11-13-3-9-16(10-4-13)29-19(27)14-5-7-15(8-6-14)23-20(21)22;1-5(2,3)4/h3-10H,11-12H2,1-2H3,(H4,21,22,23);1H3,(H,2,3,4)
InChiKey:
FSEKIHNIDBATFG-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 59721-29-8. Formula: C20H22N4O5 . CH4O3S. MW: 398.4 . 96.1. Camostat is an orally bioavailable trypsin-like protease inhibitor known to inhibit trypsin and various inflammatory proteases including plasmin, kallikrein and thrombin. Camostat has been shown to inhibit the production of TNF-alpha and monocyte chemoattractant protein-1 by monocytes and to disrupt proliferation of pancreatic stellate cells in a rat model of pancreatic fibrosis. Protease regulation via camostat has also been reported to reversibly inhibit epithelial sodium channel function in human airway epithelial cell models. It is used in the treatment of some forms of cancer and is also effective against some viral infections (such as SARS, MERS or influenza), as well as inhibiting fibrosis in liver or kidney disease or pancreatitis. It suppresses pancreatitis-induced pain in rats following oral administration and is used to treat pancreatitis and reflux esophagitis after gastrectomy. It is an inhibitor of the enzyme transmembrane serine protease TMPRSS2 and partially blocks infection by SARS-CoV and human coronavirus NL63 in HeLa cells and entry of SARS-CoV2 (COVID-19) into lung cells in vitro.
MDL:
MFCD00941410
Molecular Formula:
C20H22N4O5 . CH4O3S
Molecular Weight:
398.4 . 96.1
Package Type:
Vial
PG:
III
Precautions:
P261, P273, P280, P302+P352, P304+P340+P312, P305+P351+P338, P405, P501
Product Description:
Camostat is an orally bioavailable trypsin-like protease inhibitor known to inhibit trypsin and various inflammatory proteases including plasmin, kallikrein and thrombin. Camostat has been shown to inhibit the production of TNF-alpha and monocyte chemoattractant protein-1 by monocytes and to disrupt proliferation of pancreatic stellate cells in a rat model of pancreatic fibrosis. Protease regulation via camostat has also been reported to reversibly inhibit epithelial sodium channel function in human airway epithelial cell models. It is used in the treatment of some forms of cancer and is also effective against some viral infections (such as SARS, MERS or influenza), as well as inhibiting fibrosis in liver or kidney disease or pancreatitis. It suppresses pancreatitis-induced pain in rats following oral administration and is used to treat pancreatitis and reflux esophagitis after gastrectomy. It is an inhibitor of the enzyme transmembrane serine protease TMPRSS2 and partially blocks infection by SARS-CoV and human coronavirus NL63 in HeLa cells and entry of SARS-CoV2 (COVID-19) into lung cells in vitro.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
NC(NC1=CC=C(C(OC2=CC=C(CC(OCC(N(C)C)=O)=O)C=C2)=O)C=C1)=N.OS(=O)(C)=O
Solubility Chemicals:
Soluble in DMSO (25mg/ml), DMF (25mg/ml) or water (25mg/ml).
Transportation:
Non-hazardous
UN Nummer:
3077
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
In vivo effects of camostat mesilate on plasma kallikrein, plasma kininase II and renal kallikrein of man: G. Bonner, et al.; Arzneimittelf. 37, 535 (1987) | Suppressive effect of camostat mesilate (FOY 305) on acute experimental allergic encephalomyelitis (EAE): T. Inuzuka, et al.; Neurochem. Res. 13, 225 (1988) | Effect of camostat mesilate for the treatment of advanced diabetic nephropathy: M. Matsubara, et al.; J. Lab. Clin. Med. 116, 206 (1990) | Gabexate and camostat, synthetic proteinase inhibitors, as direct inducing factors of water and bicarbonate secretion in the isolated and blood-perfused dog pancreas: A. Horiuchi, et al.; J. Pharmacol. Exp. Ther. 252, 320 (1990) | Blocking effects of synthetic trypsin inhibitor (camostat) on pancreatic carcinogenesis in hamsters initiated with N-nitrosobis(2-oxopropyl)amine: F. Furukawa, et al.; Pancreas 9, 78 (1994) | Evaluation of anti-influenza effects of camostat in mice infected with non-adapted human influenza viruses: M.G. Lee, et al.; Arch. Virol. 141, 1979 (1996) | Camostat mesilate attenuates pancreatic fibrosis via inhibition of monocytes and pancreatic stellate cells activity: J. Gibo, et al.; Lab. Invest. 85, 75 (2005) | The proteinase inhibitor camostat mesilate suppresses pancreatic pain in rodents: H. Ishikura, et al.; Life Sci. 80, 1999 (2007) | Camostat attenuates airway epithelial sodium channel function in vivo through the inhibition of a channel-activating protease: K. Coote, et al.; J. Pharmacol. Exp. Ther. 329, 764 (2009) | Simultaneous Treatment of Human Bronchial Epithelial Cells With Serine and Cysteine Protease Inhibitors Prevents Severe Acute Respiratory Syndrome Coronavirus Entry: M. Kawase, et al.; J. Virol. 86, 6537 (2012) | The serine protease inhibitor camostat inhibits influenza virus replication and cytokine production in primary cultures of human tracheal epithelial cells: M. Yamaya, et al.; Pulm. Pharmacol. Ther. 33, 66 (2015) | Protease Inhibitors Targeting Coronavirus and Filovirus Entry: Y. Zhou, et al.; Antiviral Res. 116, 76 (2015) | SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically-proven protease inhibitor: M. Hoffmann, et al.; Cell (Epub ahead of print) (2020)