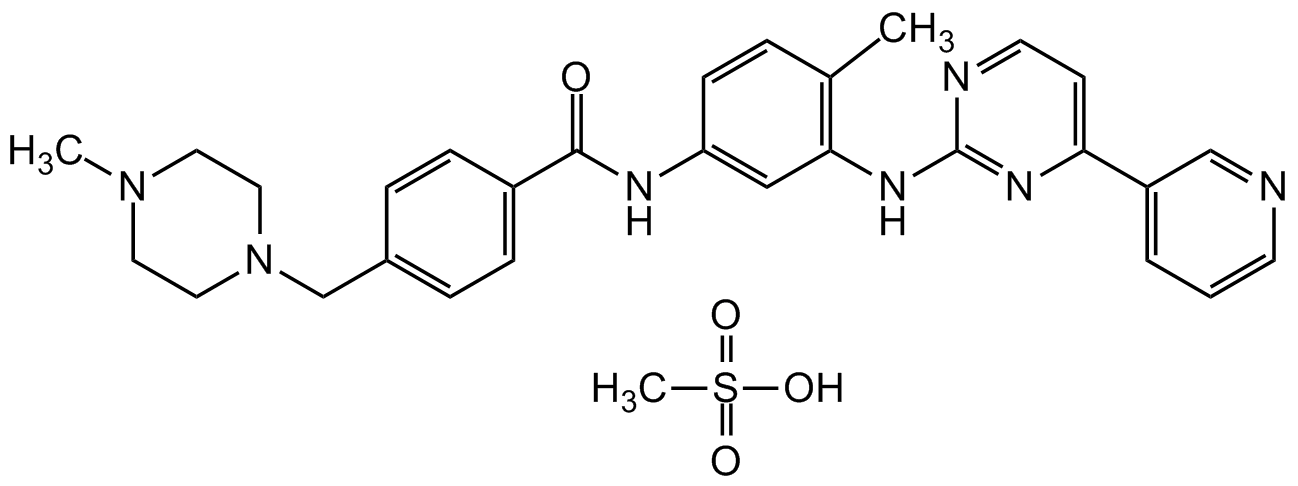
Imatinib . mesylate
| Code | Size | Price |
|---|
| AG-CR1-3725-M025 | 25 mg | £40.00 |
Quantity:
| AG-CR1-3725-M100 | 100 mg | £70.00 |
Quantity:
| AG-CR1-3725-M250 | 250 mg | £190.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
CGP-54148B; STI-571; Gleevec; Glivec
Appearance:
White to off-white solid.
CAS:
220127-57-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302
InChi:
InChI=1S/C29H31N7O.CH4O3S/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36;1-5(2,3)4/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34);1H3,(H,2,3,4)
InChiKey:
YLMAHDNUQAMNNX-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 220127-57-1. Formula: C29H31N7O . CH4SO3. MW: 493.6 . 96.1. Imatinib mesylate is a multitarget tyrosine kinase inhibitor with antineoplastic activity. It is a potent and selective inhibitor of the kinases Bcr-Abl (IC50 = 38 nM), PDGFR and c-Kit, which are encoded by the bcr-abl oncogene, as well as receptor tyrosine kinases encoded by c-kit and platelet-derived growth factor receptor (PDGFR) oncogenes, which is useful in vitro and in vivo. Imatinib inhibits ligand-stimulated autophosphorylation of PDGFR and c-Kit and the proliferation of Bcr-Abl-dependent R10(?) cells and HMC-1 cells expressing constitutively active c-Kit in a concentration-dependent manner. Formulations containing imatinib have been used in the treatment of various cancers. Specifically, it is used for chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL) that are Philadelphia chromosome-positive (Ph+), certain types of gastrointestinal stromal tumors (GIST), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), systemic mastocytosis and myelodysplastic syndrome. Abelson (Abl) kinase inhibitors have been shown to inhibit replication of Ebola virus, coxsackievirus and vaccinia virus, but at different points of the virus life cycle. Imatinib inhibits replication of SARS-CoV and MERS-CoV in vitro by a novel mechanism of blocking coronavirus virion fusion with the endosomal membrane. Abl is also a potential drug target of SARS-CoV-2 and can possible inhibit the spread of COVID-19.
MDL:
MFCD04307699
Molecular Formula:
C29H31N7O . CH4SO3
Molecular Weight:
493.6 . 96.1
Package Type:
Plastic Vial
Precautions:
P301+P312
Product Description:
Imatinib mesylate is a multitarget tyrosine kinase inhibitor with antineoplastic activity. It is a potent and selective inhibitor of the kinases Bcr-Abl (IC50 = 38 nM), PDGFR and c-Kit, which are encoded by the bcr-abl oncogene as well as receptor tyrosine kinases encoded by c-Kit and platelet-derived growth factor receptor (PDGFR) oncogenes, which is useful in vitro and in vivo. Imatinib inhibits ligand-stimulated autophosphorylation of PDGFR and c-Kit and the proliferation of Bcr-Abl-dependent R10 cells and HMC-1 cells expressing constitutively active c-Kit in a concentration-dependent manner. Formulations containing imatinib have been used in the treatment of various cancers. Specifically, it is used for chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL) that are Philadelphia chromosome-positive (Ph+), certain types of gastrointestinal stromal tumors (GIST), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), systemic mastocytosis and myelodysplastic syndrome. Abelson (Abl) kinase inhibitors have been shown to inhibit replication of Ebola virus, coxsackievirus and vaccinia virus, but at different points of the virus life cycle. Imatinib inhibits replication of SARS-CoV and MERS-CoV in vitro by a novel mechanism of blocking coronavirus virion fusion with the endosomal membrane. Abl is also a potential drug target of SARS-CoV-2 and can possible inhibit the spread of COVID-19.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
CS(O)(=O)=O.CN1CCN(CC2=CC=C(C=C2)C(=O)NC2=CC=C(C)C(NC3=NC=CC(=N3)C3=CN=CC=C3)=C2)CC1
Solubility Chemicals:
Soluble in water (30mg/ml) or DMSO (30mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Protein Kinase Modulators
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative: E: Buchdunger, et al.; Cancer Res. 56, 100 (1996) | Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells: B.J. Druker, et al.; Nat. Med. 2, 561 (1996) | Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor: M.C. Heinrich, et al.; Blood 96, 925 (2000) | Inhibition of c-kit tyrosine kinase by ima. mesylate induces apoptosis in mast cells in rheumatoid synovia: a potential approach to the treatment of arthritis: A. Juurikivi, et al.; Ann. Rheum. Dis. 64, 1126 (2005) | Repurposing of clinically developed drugs for treatment of Middle East Respiratory Syndrome coronavirus infection: J. Dyall, et al.; Antimicrob. Agents Chemother. 58, 4885 (2014) | Imatinib mesylate: C.F. Waller; Recent Results Cancer Res. 201, 1 (2014) (Review) | Imatinib: a breakthrough of targeted therapy in cancer: N. Iqbal & N. Iqbal; Chemother. Res. Pract. 2014, 357027 (2014) (Review) | Immunological off-target effects of imatinib: L. Zitvogel, et al.; Nat. Rev. Clin. Oncol. 13, 431 (2016) (Review) | Abelson Kinase Inhibitors Are Potent Inhibitors of Severe Acute Respiratory Syndrome Coronavirus and Middle East Respiratory Syndrome Coronavirus Fusion: C.M. Coleman, et al.; J. Virol. 90, 8924 (2016) | Coronavirus S protein-induced fusion is blocked prior to hemifusion by Abl kinase inhibitors: J.M. Sisk, et al.; J. Gen. Virol. 99, 619 (2018) | Expanding spectrum of anticancer drug, imatinib, in the disorders affecting brain and spinal cord: M. Kumar, et al.; Pharmacol. Res. 143, 86 (2019) (Review) | Src tyrosine kinase inhibitors: New perspectives on their immune, antiviral, and senotherapeutic potential: J. Rivera-Torres & E. San Jos?; Front. Pharmacol. 10, 110 (2019) (Review)