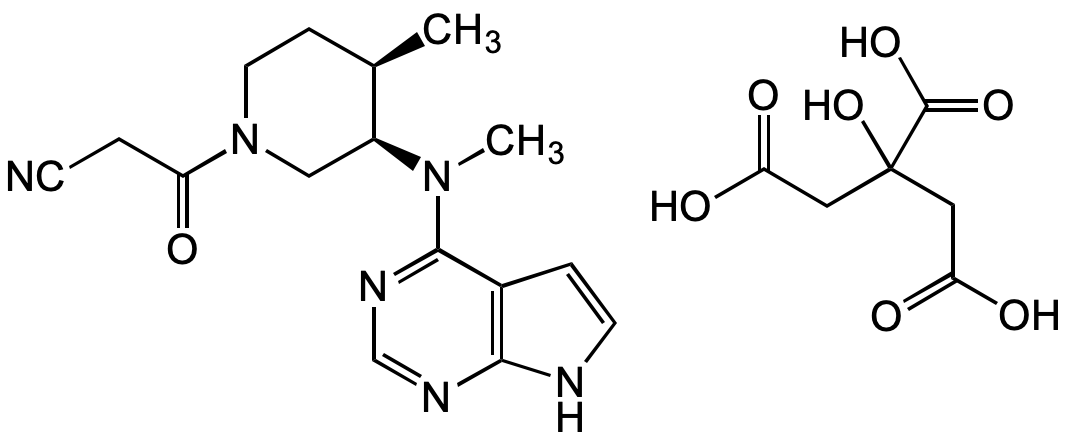
Tofacitinib . citrate
| Code | Size | Price |
|---|
| AG-CR1-3732-M005 | 5 mg | £60.00 |
Quantity:
| AG-CR1-3732-M025 | 25 mg | £110.00 |
Quantity:
| AG-CR1-3732-M100 | 100 mg | £200.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Images
Documents
Further Information
Alternate Names/Synonyms:
CP-690550; Xeljanz; Tasocitinib
Appearance:
White to off-white solid.
CAS:
540737-29-9
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS08
Handling Advice:
Keep cool and dry.Protect from moisture and oxygen.
Hazards:
H302, H360
InChi:
InChI=1S/C16H20N6O.C6H8O7/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16;7-3(8)1-6(13,5(11)12)2-4(9)10/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20);13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)/t11-,13+;/m1./s1
InChiKey:
SYIKUFDOYJFGBQ-YLAFAASESA-N
Long Description:
Chemical. CAS: 540737-29-9. Formula: C16H20N6O . C6H8O7. MW: 312.4 . 192.1. Potent cell permeable JAK3 inhibitor (IC50 = 1nM). Shows also JAK-1 inhibitory activity.
Immunosuppressive and anti-inflammatory agent.
Inhibits signaling through heterodimeric receptors associated with JAK3, JAK1, or both of them, with functional selectivity over JAK2-paired receptors. Blocks downstream STAT signaling, resulting in potent inhibition of several cytokines, including interleukins 2, 4, 7, 9, 15 and 21, which are integral to lymphocyte activation, function and proliferation.
Investigated for several autoimmune disorders including, rheumatoid arthritis and psoriasis.
Potent and selective inhibitor of HIV-1 replication and virus reactivation in vitro. It is investigated against the spread of the SARS-CoV-2 (COVID-19).
PRK1 inhibitor.
MDL:
MFCD11616529
Molecular Formula:
C16H20N6O . C6H8O7
Molecular Weight:
312.4 . 192.1
Package Type:
Vial
Precautions:
P301+P312, P308+P313
Product Description:
Potent cell permeable JAK3 inhibitor (IC50 = 1nM). Shows also JAK-1 inhibitory activity. Immunosuppressive and anti-inflammatory agent. Inhibits signaling through heterodimeric receptors associated with JAK3, JAK1, or both of them, with functional selectivity over JAK2-paired receptors. Blocks downstream STAT signaling, resulting in potent inhibition of several cytokines, including interleukins 2, 4, 7, 9, 15 and 21, which are integral to lymphocyte activation, function and proliferation. Investigated for several autoimmune disorders including, rheumatoid arthritis and psoriasis. Potent and selective inhibitor of HIV-1 replication and virus reactivation in vitro. It is investigated against the spread of the SARS-CoV-2 (COVID-19). PRK1 inhibitor.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
OC(CC(O)=O)(CC(O)=O)C(O)=O.O=C(CC#N)N1C[C@@H]([C@H](C)CC1)N(C)C2=NC=NC3=C2C=CN3
Solubility Chemicals:
Soluble in DMSO (20mg/ml) or DMF (5mg/ml). Slightly soluble in ethanol or water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor: P.S. Changelian, et al.; Science 302, 875 (2003) | Immunosuppression by the JAK3 inhibitor CP-690,550 delays rejection and significantly prolongs kidney allograft survival in nonhuman primates: D.C. Borie, et al.; Transplantation 79, 791 (2005) | Combined use of the JAK3 inhibitor CP-690,550 with mycophenolate mofetil to prevent kidney allograft rejection in nonhuman primates: D.C. Borie, et al.; Transplantation 80, 1756 (2005) | The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia: E. Kudlacz, et al.; Eur. J. Pharmacol. 582, 154 (2008) | CP-690550, a JAK3 inhibitor as an immunosuppressant for the treatment of rheumatoid arthritis, transplant rejection, psoriasis and other immune-mediated disorders: K. West; Curr. Opin. Investig. Drugs 10, 491 (2009) (Review) | Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection: M.E. Flanagan, et al.; J. Med. Chem. 53, 8468 (2010) | CP-690,550, a therapeutic agent, inhibits cytokine-mediated Jak3 activation and proliferation of T cells from patients with ATL and HAM/TSP: W. Ju, et al.; Blood 117, 1938 (2011) | Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550): K. Ghoreschi, et al.; J. Immunol. 186, 4234 (2011) | Inhibitory effects of the JAK inhibitor CP690,550 on human CD4(+) T lymphocyte cytokine production: K. Migita, et al.; BMC Immunol. 12, 51 (2011) | JAK inhibitor tofacitinib for treating rheumatoid arthritis: from basic to clinical: Y. Tanaka & K. Yamaoka; Mod. Rheumatol. 23, 415 (2013) (Review) | Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro: C. Gavegnano, et al.; Antimicrob. Agents Chemother. 58, 1977 (2014) | Tofacitinib, a JAK inhibitor, inhibits human B cell activation in vitro: S.P. Wang, et al.; Ann. Rheum. Dis. 73, 2213 (2014) | Crystal structures of PRK1 in complex with the clinical compounds lestaurtinib and tofacitinib reveal ligand induced conformational changes: P. Chamberlain, et al.; PLos One 9, e103638 (2014) | The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis: D.L. Boyle, et al.; Ann. Rheum. Dis. 74, 1311 (2015) | Matrix stiffness regulates endosomal escape of uropathogenic E. coli: S. Moorthy, et al.; Cell Microbiol. 22, e13196 (2020) | Case Report of a SARS-CoV-2 Infection in a Patient With Ulcerative Colitis on Tofacitinib: J. Jacobs, et al.; Inflamm. Bowel Dis. (Epub ahead of print) (2020)