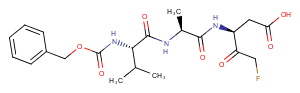
Z-VAD-FMK
Product Code:
TAR-T7020
TAR-T7020
Regulatory Status:
RUO
RUO
Shipping:
cool pack
cool pack
Storage:
-20℃
-20℃
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| TAR-T7020-1mg | 1mg | £168.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T7020-5mg | 5mg | £277.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T7020-1mL | 1 mL * 10 mM (in DMSO) | £298.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T7020-10mg | 10mg | £422.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
| TAR-T7020-25mg | 25mg | £630.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Special offer! Add £1 to your order to get a TargetMol CCK-8 Kit. Read more here. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: United States.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Bioactivity:
Z-VAD-FMK (Caspase Inhibitor VI) is an irreversible pan-caspase inhibitor.
Biological Applications:
Z-VAD-FMK, as a broad-spectrum inhibitor of Caspase, plays a crucial role in apoptosis research. Numerous preclinical studies, both in vitro and in vivo, indicate that Caspase primarily acts as an inflammatory and apoptotic mediator in various pathologies. Consequently, several Caspase inhibitors have been patented for their anti-inflammatory and apoptotic functions. However, due to factors such as drug toxicity, their application is currently limited to preclinical research. Although some studies propose novel therapeutic approaches using nanoparticle delivery systems and CRISPR/Cas9 gene editing to improve drug delivery and reduce drug-induced toxicity, targeting individual Caspases separately, these remain short-term solutions. Because the lack of Caspase activity can increase the crosstalk between cell death and inflammatory pathways, there are concerns about the long-term efficacy of Caspase inhibitors. If inhibitors increase the risk of cell death and inflammatory reactions, they may exacerbate diseases. Therefore, it is crucial to clearly determine the specific mechanisms of action of Caspase inhibitors in preclinical models.
CAS:
161401-82-7
Formula:
C21H28FN3O7
Long Description:
Z-VAD-FMK (Caspase Inhibitor VI) is an irreversible pan-caspase inhibitor.
Mechanism of Action:
Z-VAD (OMe)-FMK and Z-VAD (OH)-FMK are both caspase inhibitors, but the differences in their chemical structures lead to distinct properties and modes of action. Z-VAD (OMe)-FMK is a cell-permeable, irreversible caspase inhibitor that can inhibit caspases and apoptosis in tumor cells in vitro. The methyl ester compound undergoes hydrolysis by endogenous esterase activity upon entering the cell, generating the biologically active form. Therefore, pre-treatment with esterase is required when using it with isolated, purified, or recombinant caspases. On the other hand, Z-VAD (OH)-FMK is a carboxylic acid compound that can be directly added to cell culture media without the need for esterase pre-treatment.
Molecular Weight:
453.467
Pathway:
Apoptosis; Proteases/Proteasome
Purity:
0.9944
Research Area:
Neurological disorders (epilepsy, Parkinson's, etc.), Inflammatory diseases (rheumatoid arthritis, sepsis), AIDS, Obesity, diabetes, Liver disease, Breast cancer, lung cancer, etc.
SMILES:
CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CF
Target:
Caspase
References
Qiu C, Shen X, Lu H, et al.Combination therapy with HSP90 inhibitors and piperlongumine promotes ROS-mediated ER stress in colon cancer cells.Cell Death Discovery.2023, 9(1): 375.
Wang X, Ji Y, Qi J, et al.Mitochondrial carrier 1 (MTCH1) governs ferroptosis by triggering the FoxO1-GPX4 axis-mediated retrograde signaling in cervical cancer cells.Cell Death & Disease.2023, 14(8): 1-13.
Garcia-Calvo M, et al. J Biol Chem. 1998, 273(49), 32608-32613.
Hu G, Cui Z, Chen X, et al.Suppressing Mesenchymal Stromal Cell Ferroptosis Via Targeting a Metabolism?Epigenetics Axis Corrects their Poor Retention and Insufficient Healing Benefits in the Injured Liver Milieu.Advanced Science.2023: 2206439.
Zhu X, Huang N, Ji Y, et al.Brusatol induces ferroptosis in oesophageal squamous cell carcinoma by repressing GSH synthesis and increasing the labile iron pool via inhibition of the NRF2 pathway.Biomedicine & Pharmacotherapy.2023, 167: 115567.
Huang F, Liang J, Lin Y, et al.Repurposing of Ibrutinib and Quizartinib as potent inhibitors of necroptosis.Communications Biology.2023, 6(1): 972.
Shao H, Xu L, Li G, et al.Analysis on benzothiazole necroptosis inhibitors with chiral substitutions in the solvent-accessible region of RIP kinase domain.Bioorganic Chemistry.2023: 106647.
Sun Y, He L, Wang T, et al. Activation of p62-Keap1-Nrf2 Pathway Protects 6-Hydroxydopamine-Induced Ferroptosis in Dopaminergic Cells[J]. Molecular Neurobiology. 2020, 57(11): 4628-4641.
Yan C, Zheng L, Jiang S, et al.Exhaustion-associated cholesterol deficiency dampens the cytotoxic arm of antitumor immunity.Cancer Cell.2023
Xue J, Gruber F, Tschachler E, et al. Crosstalk between oxidative stress, autophagy and apoptosis in Hemoporfin Photodynamic Therapy treated human umbilical vein endothelial cells[J]. Photodiagnosis and Photodynamic Therapy. 2020: 102137.
Ning X, Qi H, Yuan Y, et al. Identification of a new small molecule that initiates ferroptosis in cancer cells by inhibiting the system Xc? to deplete GSH. European Journal of Pharmacology. 2022: 175304.
Zeng H, Xie H, Ma Q, et al.Identification of N-(3-(methyl (3-(orotic amido) propyl) amino) propyl) oleanolamide as a novel topoisomerase I catalytic inhibitor by rational design, molecular dynamics simulation, and biological evaluation.Bioorganic Chemistry.2023: 106734.
Sun Y, Xu L, Shao H, et al. Discovery of a Trifluoromethoxy Cyclopentanone Benzothiazole Receptor-Interacting Protein Kinase 1 Inhibitor as the Treatment for Alzheimer?s Disease. Journal of Medicinal Chemistry. 2022
Wang F, Xie M, Chen P, et al. Homoharringtonine combined with cladribine and aclarubicin (HCA) in acute myeloid leukemia: A new regimen of conventional drugs and its mechanism. Oxidative Medicine and Cellular Longevity. 2022
Xue J, Gruber F, Tschachler E, et al. Crosstalk between oxidative stress, autophagy and apoptosis in Hemoporfin Photodynamic Therapy treated human umbilical vein endothelial cells. Photodiagnosis and Photodynamic Therapy. 2020: 102137.
Tschuck J, Theilacker L, Rothenaigner I, et al.Farnesoid X receptor activation by bile acids suppresses lipid peroxidation and ferroptosis.Nature Communications.2023, 14(1): 6908.
Tian T, Xie X, Yi W, et al.FBXO38 mediates FGL1 ubiquitination and degradation to enhance cancer immunity and suppress inflammation.Cell Reports.2023, 42(11).
Su G, Yang W, Wang S, et al. SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases IL-1? and IL-18. Biochemical and Biophysical Research Communications. 2021, 561: 33-39.
Wang S, Wang Z, Wang X, et al. Humanized cerebral organoids-based ischemic stroke model for discovering of potential anti-stroke agents. Acta Pharmacologica Sinica. 2022: 1-11.
Sun Y, He L, Wang T, et al. Activation of p62-Keap1-Nrf2 Pathway Protects 6-Hydroxydopamine-Induced Ferroptosis in Dopaminergic Cells. Molecular Neurobiology. 2020, 57(11): 4628-4641.