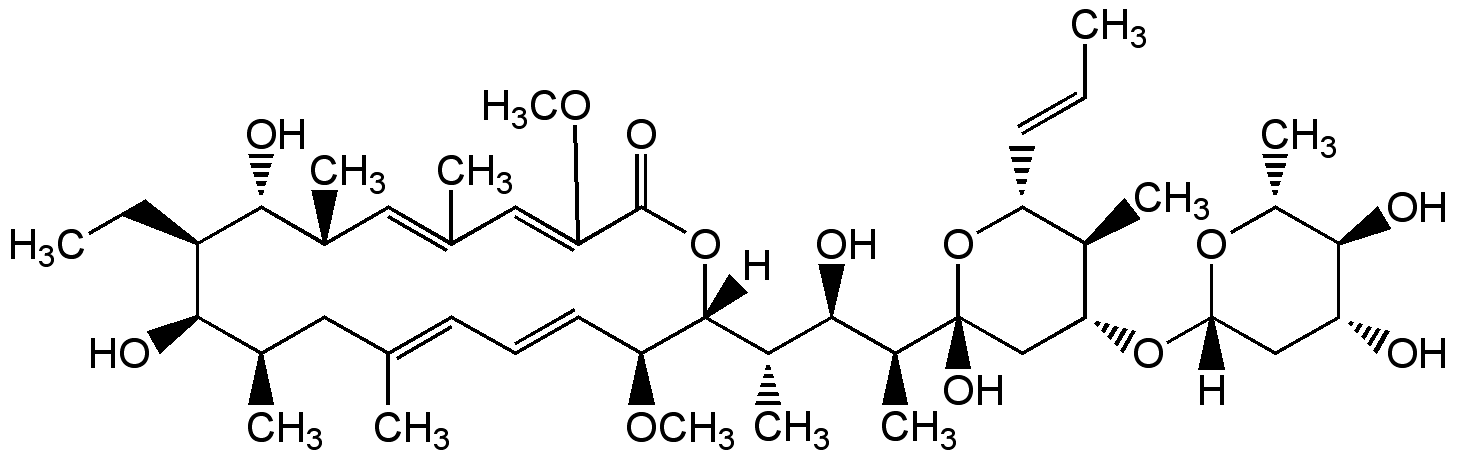
Concanamycin C
Product Code:
BVT-0254
BVT-0254
Regulatory Status:
RUO
RUO
Shipping:
20°C
20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| BVT-0254-C025 | 25 ug | £95.00 |
Quantity:
| BVT-0254-C100 | 100 ug | £190.00 |
Quantity:
| BVT-0254-C500 | 500 ug | £525.00 |
Quantity:
| BVT-0254-M001 | 1 mg | £740.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
Typical lead time: 10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
4'-O-De(aminocarbonyl)concanamycin A
Appearance:
White to off-white solid.
CAS:
81552-34-3
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06
Handling Advice:
Protect from light when in solution.
Hazards:
H300, H310, H319, H332
InChi:
InChI=1S/C45H74O13/c1-13-16-34-28(7)37(56-38-22-33(46)42(50)31(10)55-38)23-45(52,58-34)30(9)41(49)29(8)43-35(53-11)18-15-17-24(3)19-26(5)39(47)32(14-2)40(48)27(6)20-25(4)21-36(54-12)44(51)57-43/h13,15-18,20-21,26-35,37-43,46-50,52H,14,19,22-23H2,1-12H3/b16-13+,18-15+,24-17+,25-20-,36-21+/t26-,27-,28-,29+,30+,31-,32-,33-,34-,35?,37-,38+,39-,40-,41-,42-,43-,45-/m1/s1
InChiKey:
XKYYLWWOGLVPOR-PLWOKYFKSA-N
Long Description:
Chemical. CAS: 81552-34-3. Formula: C45H74O13. MW: 823.1. Isolated from Streptomyces sp. Antibiotic. Vacuolar-type H+-ATPase inhibitor. Inhibitor of autophagic degradation by rising lysosomal pH and thus inactivating the lysosomal acid hydrolases. Anti-osteoporotic. Antiviral. Immunosuppressive. Antifungal and anti-yeast.
MDL:
MFCD00216692
Molecular Formula:
C45H74O13
Molecular Weight:
823.1
Package Type:
Plastic Vial
PG:
III
Precautions:
P261, P262, P280, P301, P310, P302, P350, P312
Product Description:
Antibiotic. Vacuolar-type H+-ATPase inhibitor. Inhibitor of autophagic degradation by rising lysosomal pH and thus inactivating the lysosomal acid hydrolases. Anti-osteoporotic. Antiviral. Immunosuppressive. Antifungal and anti-yeast.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
[H][C@@]1(C[C@@H](O)[C@H](O)[C@@H](C)O1)O[C@@H]1C[C@@](O)(O[C@H](C=CC)[C@H]1C)[C@@H](C)[C@H](O)[C@H](C)[C@@]1([H])OC(=O)C(OC)=CC(C)=C[C@@H](C)[C@@H](O)[C@H](CC)[C@H](O)[C@H](C)CC(C)=CC=C[C@@H]1OC
Solubility Chemicals:
Soluble in DMSO, methanol, chloroform or acetonitrile; insoluble in water.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
Documents
References
Structures of concanamycins B and C: H. Kinashi, et al.; J. Antibiot. (Tokyo) 35, 1618 (1982) | Isolation and characterization of concanamycins A, B and C: H. Kinashi, et al.; J. Antibiot. (Tokyo) 37, 1333 (1984) | Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases: S. Drose, et al.; Biochemistry 32, 3902 (1993) | Disruption of vma-1, the gene encoding the catalytic subunit of the vacuolar H(+)-ATPase, causes severe morphological changes in Neurospora crassa: E.J. Bowman, et al.; J. Biol. Chem. 275, 167 (2000) | Semisynthetic derivatives of concanamycin A and C, as inhibitors of V- and P-type ATPases: structure-activity investigations and developments of photoaffinity probes: S. Drose, et al.; Biochemistry 40, 2816 (2001) | Inhibitors of V-ATPase: old and new players: M. Huss, et al.; J. Exp. Biol. 212, 341 (2009)