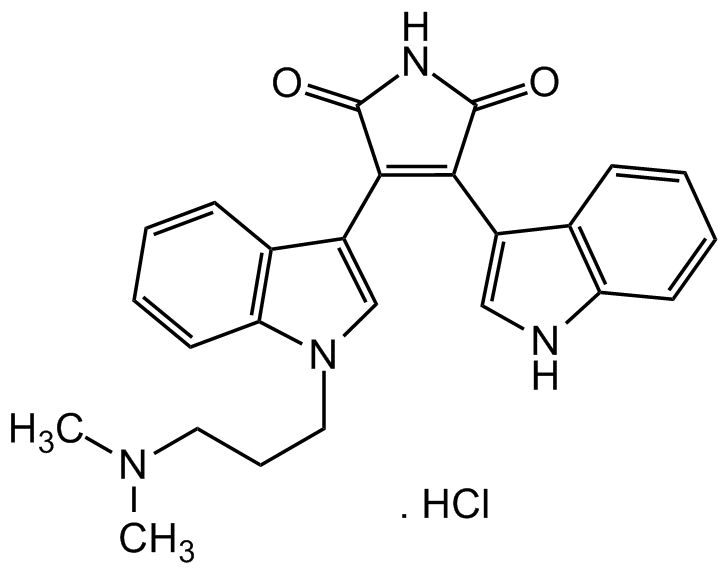
Bisindolylmaleimide I . Hydrochloride
| Code | Size | Price |
|---|
| AG-CR1-0110-M001 | 1 mg | £60.00 |
Quantity:
| AG-CR1-0110-M005 | 5 mg | £160.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
-20°C
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
GF 109203X; G? 6850; BIM I
Appearance:
Orange to red powder.
CAS:
176504-36-2
EClass:
32160000
Form (Short):
liquid
InChi:
InChI=1S/C25H24N4O2.ClH/c1-28(2)12-7-13-29-15-19(17-9-4-6-11-21(17)29)23-22(24(30)27-25(23)31)18-14-26-20-10-5-3-8-16(18)20;/h3-6,8-11,14-15,26H,7,12-13H2,1-2H3,(H,27,30,31);1H
InChiKey:
XRAMWNCMYJHGGH-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 176504-36-2. Formula: C25H24N4O2 . HCl. MW: 412.5 . 36.5. Cell permeable kinase inhibitor with improved selectivity for protein kinase C (PKC) (Ki = 10 nM). Competitive inhibitor for the ATP-binding site of PKC. Anti-inflammatory. Binds to P-glycoprotein. Telomerase activity inhibitor. Potent glycogen synthase kinase-3 (GSK-3) inhibitor. Necrosis inhibitor. Blocks hERG potassium channels. Promotes osteoblastogenesis in human mesenchymal stem cells.
MDL:
MFCD00236429
Molecular Formula:
C25H24N4O2 . HCl
Molecular Weight:
412.5 . 36.5
Package Type:
Vial
Product Description:
Cell permeable kinase inhibitor with improved selectivity for protein kinase C (PKC) (Ki = 10 nM) [1]. Competitive inhibitor for the ATP-binding site of PKC [1]. Anti-inflammatory [2]. Binds to P-glycoprotein [3]. Telomerase activity inhibitor [4]. Potent glycogen synthase kinase-3 (GSK-3) inhibitor [5]. Necrosis inhibitor [6]. Blocks hERG potassium channels [7-9]. Promotes osteoblastogenesis in human mesenchymal stem cells [10].
Purity:
>98% (NMR)
SMILES:
Cl.CN(C)CCCN1C=C(C2=C1C=CC=C2)C1=C(C(=O)NC1=O)C1=CNC2=C1C=CC=C2
Solubility Chemicals:
Soluble in DMSO or methanol. Slightly soluble in water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Store solutions at -20°C in the dark.
References
The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C: D. Toullec, et al.; J. Biol. Chem. 266, 15771 (1991) | Anti-inflammatory properties of the protein kinase C inhibitor, 3-[1-[3- (dimethylamino)propyl]-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H- pyrrole-2,5- dione monohydrochloride (GF109203X) in the PMA-mouse ear edema model: S. Kuchera, et al.; Agents Actions 39, C169 (1993) | Effects of the selective bisindolylmaleimide protein kinase C inhibitor GF 109203X on P-glycoprotein-mediated multidrug resistance: V. Gekeler, et al.; Br. J. Cancer 74, 897 (1996) | Inhibition of telomerase activity by PKC inhibitors in human nasopharyngeal cancer cells in culture: W.C. Ku, et al.; BBRC 241, 730 (1997) | The protein kinase C inhibitors bisindolylmaleimide I (GF 109203x) and IX (Ro 31-8220) are potent inhibitors of glycogen synthase kinase-3 activity: I. Hers, et al.; FEBS Lett. 460, 433 (1999) | Bisindolylmaleimide I and V inhibit necrosis induced by oxidative stress in a variety of cells including neurons: R. Asakai, et al.; Neurosci. Res. 44, 297 (2002) | Direct block of hERG potassium channels by the protein kinase C inhibitor bisindolylmaleimide I (GF109203X): D. Thomas, et al.; Cardiovasc. Res. 64, 467 (2004) | The protein kinase C inhibitor, bisindolylmaleimide (I), inhibits voltage-dependent K+ channels in coronary arterial smooth muscle cells: W.S. Park, et al.; Life Sci. 77, 512 (2005) | Effects of the PKC inhibitors chelerythrine and bisindolylmaleimide I (GF 109203X) on delayed rectifier K(+) currents: G. Harmati, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 383, 141 (2011) | Bisindolylmaleimide I enhances osteogenic differentiation: F. Zhou, et al.; Prot. Cell 3, 311 (2012)
Related Products
| Product Name | Product Code | Supplier | (S)-CR8 | AG-CR1-0040 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (-)-Indolactam V | AG-CR1-0041 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6BIO | AG-CR1-0056 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bisindolylmaleimide IX . methansulfonate [Ro 31-8220] | AG-CR1-0111 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bisindolylmaleimide III | AG-CR1-0112 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Leucettine L41 | AG-MR-C0023 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||