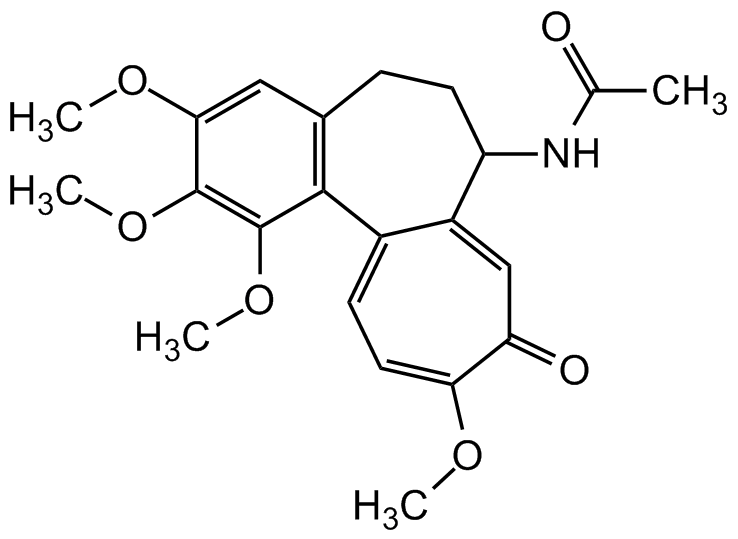
Colchicine
Product Code: AG-CN2-0048
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0048-M500 | 500 mg | £70.00 |
Quantity:
| AG-CN2-0048-G001 | 1 g | £110.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4deg;C
Images
Documents
Further Information
Alternate Names/Synonyms:
NSC 757; EINECS 200-598-5
Appearance:
White to off-white powder.
CAS:
64-86-8
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS06,GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H300, H340
InChi:
InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)
InChiKey:
IAKHMKGGTNLKSZ-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 64-86-8. Formula: C22H25NO6. MW: 399.4. Isolated from Colchicum autumnale. Anti-cancer compound. Microtubule assembly inhibitor. Depolymerizes microtubules and limits microtubule formation (inactivates spindle fibre formation). Inhibits mitosis during cell division at metaphase by inhibiting spindle formation. Anti-inflammatory compound. Suppresses monosodium urate crystal-induced NLRP3/NALP3 inflammasome-driven caspase-1 activation, IL-1beta processing and release, and L-selectin expression on neutrophils at micromolar concentrations. Blocks the release of a crystal-derived chemotactic factor from neutrophil lysosomes, blocks neutrophil adhesion to, and inhibits monosodium urate crystal-induced production of superoxide anions from neutrophils at nanomolar concentrations. Drug used in treatment of gout, familial Mediterranean fever, pericarditis and Behect's disease. Investigated for its anti-cancer activity. It has a narrow therapeutic index with no clear-cut distinction between nontoxic, toxic and lethal doses, causing substantial confusion among clinicians. Apoptosis inducer in a variety of normal and tumor cell lines. Inhibitor of autophagosome-lysosome fusion. Inhibits acetylated a-tubulin mediated dynein dependent transport of mitochondria and subsequent apposition of ASC on mitochondria to NLRP3 on the endoplasmic reticulum in vitro and in vivo.
MDL:
MFCD00078484
Molecular Formula:
C22H25NO6
Molecular Weight:
399.4
Package Type:
Vial
PG:
III
Precautions:
P201, P202, P281, P301, P310, P308, P313
Product Description:
Anti-cancer compound [5]. Microtubule assembly inhibitor. Depolymerizes microtubules and limits microtubule formation (inactivates spindle fibre formation) [1, 2, 5, 6]. Inhibits mitosis during cell division at metaphase by inhibiting spindle formation [6]. Anti-inflammatory compound [7, 9]. Suppresses monosodium urate crystal-induced NLRP3/NALP3 inflammasome-driven caspase-1 activation, IL-1beta processing and release, and L-selectin expression on neutrophils at micromolar concentrations. Blocks the release of a crystal-derived chemotactic factor from neutrophil lysosomes, blocks neutrophil adhesion to, and inhibits monosodium urate crystal-induced production of superoxide anions from neutrophils at nanomolar concentrations [7]. Drug used in treatment of gout, familial Mediterranean fever, pericarditis and Behect's disease. Investigated for its anti-cancer activity [5, 8, 9]. It has a narrow therapeutic index with no clear-cut distinction between nontoxic, toxic and lethal doses, causing substantial confusion among clinicians [8]. Apoptosis inducer in a variety of normal and tumor cell lines [3, 4]. Inhibitor of autophagosome-lysosome fusion. Inhibits acetylated a-tubulin mediated dynein dependent transport of mitochondria and subsequent apposition of ASC on mitochondria to NLRP3 on the endoplasmic reticulum in vitro and in vivo.
Purity:
>97%
Signal word:
Danger
SMILES:
COC1=CC2=C(C(OC)=C1OC)C1=CC=C(OC)C(=O)C=C1C(CC2)NC(C)=O
Solubility Chemicals:
Soluble in 100% ethanol (>100mg/ml), DMSO (>100mg/ml), water or DMF.
Source / Host:
Isolated from Colchicum autumnale.
Transportation:
Excepted Quantity
UN Nummer:
UN 3462
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Interactions of colchicine with tubulin: S.B. Hastie; Pharmacol. Ther. 51, 377 (1991) (Review) | Analysis of the colchicine-binding site of beta-tubulin: R.G. Burns; FEBS Lett. 297, 205 (1992) (Review) | Colchicine induces apoptosis in cerebellar granule cells: E. Bonfoco, et al.; Exp. Cell. Res. 218, 189 (1995) | Sustained JNK activation induces endothelial apoptosis: studies with colchicine and shear stress: Y.L. Hu, et al.; Am. J. Physiol. 277, H1593 (1999) | Microtubules as a target for anticancer drugs: M.A. Jordan & L. Wilson; Nat. Rev. Cancer 4, 253 (2004) | Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin: B. Bhattacharyya, et al.; Med. Res. Rev. 28, 155 (2008) (Review) | Colchicine: its mechanism of action and efficacy in crystal-induced inflammation: G. Nuki; Curr. Rheumatol. Rep. 10, 218 (2008) (Review) | Colchicine poisoning: the dark side of an ancient drug: Y. Finkelstein, et al.; Clin. Toxicol. (Phila). 48, 407 (2010) (Review) | Colchicine for the treatment of gout: P. Richette & T. Bardin; Expert Opin. Pharmacother. 11, 2933 (2010) (Review) | Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome: T. Misawa, et al.; Nat. Immunol. 14, 454 (2013)
Related Products
| Product Name | Product Code | Supplier | Thiocolchicoside | AG-CN2-0076 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SIN-1 chloride | AG-CR1-0027 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Colcemid | AG-CR1-3567 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||