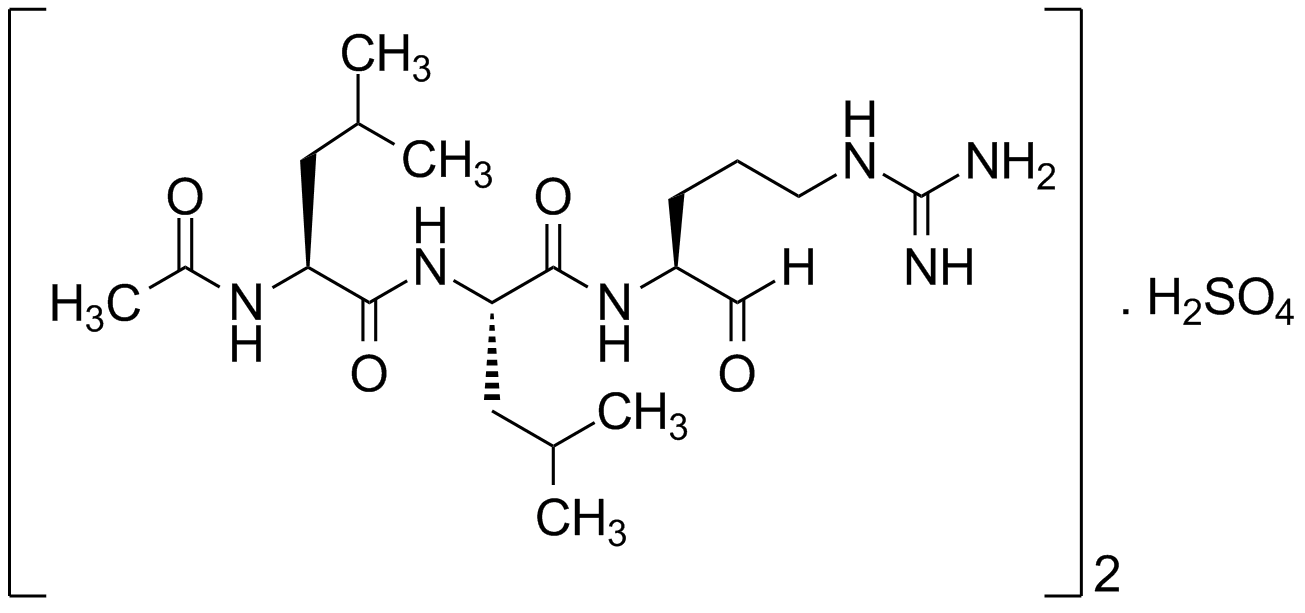
Leupeptin . hemisulfate
Product Code: AG-CP3-7000
Product Group: Antibiotics and Other Antimicrobials
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CP3-7000-M005 | 5 mg | £45.00 |
Quantity:
| AG-CP3-7000-M025 | 25 mg | £95.00 |
Quantity:
| AG-CP3-7000-M100 | 100 mg | £270.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Acetyl-L-leucyl-L-leucyl-L-argininal . hemisulfate
Appearance:
White to off-white powder.
CAS:
103476-89-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
1S/2C20H38N6O4.H2O4S/c2*1-12(2)?9-16(24-14(5)?28)?19(30)?26-17(10-13(3)?4)?18(29)?25-15(11-27)?7-6-8-23-20(21)?22;1-5(2,3)?4/h2*11-13,15-17H,6-10H2,1-5H3,(H,24,28)?(H,25,29)?(H,26,30)?(H4,21,22,23)?;(H2,1,2,3,4)?/t2*15-,16-,17-;/m00./s1
InChiKey:
CIPMKIHUGVGQTG-VFFZMTJFSA-N
Long Description:
Chemical. CAS: 103476-89-7. Formula: C20H38N6O4 . 0.5 H2SO4. MW: 426.6 . 49.0. Synthetic. Potent, competitive and reversible cysteine, serine and threonine protease inhibitor. Inhibits calpain, kallikrein, trypsin, plasmin, papain and cathepsin B. Does not inhibit pepsin, elastase, renin, cathepsins A and D, thrombin or alpha-chymotrypsin. Used to protect against hearing loss caused by acoustic overstimulation or the ototoxic antibiotic gentamicin. Shown to inhibit activation-induced programmed cell death. Antioxidant and anti-inflammatory agent. Widely used as a protein purification tool to prevent proteases present in tissue samples from degrading the protein of interest.
MDL:
MFCD00037012
Molecular Formula:
C20H38N6O4 . 0.5 H2SO4
Molecular Weight:
426.6 . 49.0
Other data:
Leupeptin gives multiple peaks on HPLC due to equilibria among three forms in solution. Purity determined using three main peaks. Majority of contaminating peptide is racemized leupeptin.
Package Type:
Vial
Product Description:
Potent, competitive and reversible cysteine, serine and threonine protease inhibitor. Inhibits calpain, kallikrein, trypsin, plasmin, papain and cathepsin B. Does not inhibit pepsin, elastase, renin, cathepsins A and D, thrombin or alpha-chymotrypsin. Used to protect against hearing loss caused by acoustic overstimulation or the ototoxic antibiotic gentamicin. Shown to inhibit activation-induced programmed cell death. Antioxidant and anti-inflammatory agent. Widely used as a protein purification tool to prevent proteases present in tissue samples from degrading the protein of interest. Molecular docking and molecular dynamics simulation of leupeptin with the transmembrane serine protease TMPRSS2, show strong interactions with the key amino acids Ser186, His41 and Asp180 of catalytic triad present in the active site of TMPRSS2. This interaction causes the inhibition of the function of TMPRSS2, which is essential for SARS-CoV-2 viral entry and infection. As an inhibitor of TMPRSS2 this agent could be repurposed for treatment of COVID-19.
Purity:
>97% (HPLC)
Sequence:
Ac-Leu-Leu-Arginal
SMILES:
OS(O)(=O)=O.[H]C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(C)=O.[H]C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(C)=O
Solubility Chemicals:
Soluble in ethanol, methanol or water.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Leupeptins, new protease inhibitors from actinomycetes: T. Aoyagi, et al.; J. Antibiot. (Tokyo) 22, 283 (1969) | Biological activities of leupeptins: T. Aoyagi; J. Antibiot. (Tokyo) 22, 558 (1969) | Mechanism of association of a specific aldehyde inhibitor, leupeptin, with bovine trypsin: H. Kuramochi, et al.; J. Biochem. 86, 1403 (1979) | The slow, tight-binding inhibition of cathepsin B by leupeptin. A hysteretic effect: A. Baici, et al.; Eur. J. Biochem. 129, 33 (1982) | Leupeptin, a thiol proteinase inhibitor, causes a selective impairment of spatial maze performance in rats: U. Staubli, et al.; Behav. Neural. Biol. 40, 58 (1984) | Leupeptin selectively inhibits human platelet responses induced by thrombin and trypsin; a role for proteolytic activation of phospholipase C: M. Ruggiero & E.G. Lapetina; BBRC 131, 1198 (1985) | Protease-inhibitory activities of leupeptin analogues: T. Saino, et al.; J. Antibiot. (Tokyo) 41, 220 (1988) | New leupeptin analogues: synthesis and inhibition data: R.M. McConnell, et al.; J. Med. Chem. 33, 86 (1990) | Developing selective inhibitors of calpain: K.K. Wang, et al.; TIPS 11, 139 (1990) | Cell-penetrating inhibitors of calpain: S. Mehdi, et al.; TIBS 16, 150 (1991) | Inhibition studies of some serine and thiol proteinases by new leupeptin analogues: R.M. McConnell, et al.; J. Med. Chem. 36, 1084 (1993) | Inhibition of activation-induced programmed cell death and restoration of defective immune responses of HIV+ donors by cysteine protease inhibitors: A. Sarin, et al.; J. Immunol. 153, 862 (1994) | Inhibition of mu and delta opioid receptor ligand binding by the peptide aldehyde protease inhibitor, leupeptin: K.H. Christoffers, et al.; Regul. Pept. 105, 9 (2002) | In vitro antioxidant properties of calpain inhibitors: leupeptin and calpain inhibitor-1: C. Perrin, et al.; Cell. Mol. Biol. (Noisy-le-grand) 48, OL267 (2002) | Inhibitors of cathepsin B: F. Frlan & S. Gobec; Curr. Med. Chem. 13, 2309 (2006) | Leupeptin, a calpain inhibitor, protects inner ear hair cells from aminoglycoside ototoxicity: J. Momiyama, et al.; Tohoku J. Exp. Med. 209, 89 (2006) | Leupeptin reduces impulse noise induced hearing loss: H. Gavriel, et al.; J. Occup. Med. Toxicol. 6, 38 (2011) | Strong Binding of Leupeptin with TMPRSS2 Protease May Be an Alternative to Camostat and Nafamostat for SARS-CoV-2 Repurposed Drug: Evaluation from Molecular Docking and Molecular Dynamics Simulations: J. Ramakrishnan, et al.; Appl. Biochem. Biotech. (epub ahead of print) (2021)
Related Products
| Product Name | Product Code | Supplier | Amastatin . hydrochloride | AG-CP3-7003 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AEBSF . hydrochloride | AG-CR1-3610 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||