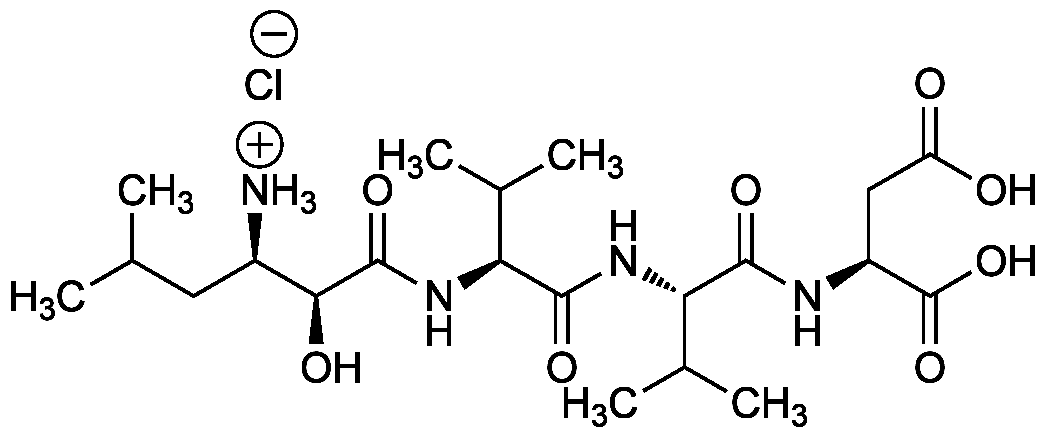
Amastatin . hydrochloride
| Code | Size | Price |
|---|
| AG-CP3-7003-M001 | 1 mg | £50.00 |
Quantity:
| AG-CP3-7003-M005 | 5 mg | £145.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
[(2S,3R)-3-Amino-2-hydroxy-5-methyl-hexanoyl]-Val-Val-Asp-OH . HCl
Appearance:
White to off-white powder.
CAS:
100938-10-1
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from moisture.
InChi:
InChI=1S/C21H38N4O8.ClH/c1-9(2)7-12(22)17(28)20(31)25-16(11(5)6)19(30)24-15(10(3)4)18(29)23-13(21(32)33)8-14(26)27;/h9-13,15-17,28H,7-8,22H2,1-6H3,(H,23,29)(H,24,30)(H,25,31)(H,26,27)(H,32,33);1H/t12-,13+,15+,16+,17+;/m1./s1
InChiKey:
GBDUPCKQTDKNLS-PORDUOSCSA-N
Long Description:
Chemical. CAS: 100938-10-1. Formula: C21H38N4O8 . HCl. MW: 474.6 . 36.5. Synthetic. Slow, tight binding and competitive aminopeptidase (AP) inhibitor. Inhibits cytosolic leucine aminopeptidase, microsomal aminopeptidase M and bacterial leucine aminopeptidase, human serum aminopeptidase A (AP-A), aminopeptidase N (AP-N), tyrosine aminopeptidase, but not aminopeptidase B (AP-B). Amastatin is without effect on trypsin, papain, chymotrypsin, elastase, pepsin or thermolysin. Inhibits completely the Suc-Ala-Ala-Pro-Leu-pNA amidolytic enzyme. Slightly inhibits the formation of angiotensin III (Ang III) from Angiotensin II through AP-A, but significantly increases the potency of angiotensin III and-angiotensin I. Moderate inhibitor of mitochondrial intermediate peptidase (MIP). Weak inhibitor of simian immunodeficiency virus protease (SIV-PR).
MDL:
MFCD00150636
Molecular Formula:
C21H38N4O8 . HCl
Molecular Weight:
474.6 . 36.5
Package Type:
Vial
Product Description:
Slow, tight binding and competitive aminopeptidase (AP) inhibitor. Inhibits cytosolic leucine aminopeptidase, microsomal aminopeptidase M and bacterial leucine aminopeptidase, human serum aminopeptidase A (AP-A), aminopeptidase N (AP-N), tyrosine aminopeptidase, but not aminopeptidase B (AP-B). Amastatin is without effect on trypsin, papain, chymotrypsin, elastase, pepsin or thermolysin. Inhibits completely the Suc-Ala-Ala-Pro-Leu-pNA amidolytic enzyme. Slightly inhibits the formation of angiotensin III (Ang III) from Angiotensin II through AP-A, but significantly increases the potency of angiotensin III and [des-Asp1]-angiotensin I. Moderate inhibitor of mitochondrial intermediate peptidase (MIP). Weak inhibitor of simian immunodeficiency virus protease (SIV-PR). ANPEP (aminopeptidase N) is a host receptor targeted by porcine epidemic diarrhoea virus, human coronavirus 229E, feline coronavirus, canine coronavirus, transmissible gastroenteritis virus and infectious bronchitis virus. These viruses all belong to coronaviridae. ANPEP is therefore investigated as a potential target for SARS-CoV-2 infections.
Purity:
>98% (HPLC)
SMILES:
[Cl-].CC(C)C[C@@H]([NH3+])[C@H](O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O
Solubility Chemicals:
Soluble in DMF, ethanol, DMSO or aqueous solvents (PBS).
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Amastatin, an inhibitor of aminopeptidase A, produced by actinomycetes: T. Aoyagi, et al.; J. Antibiot. 31, 636 (1978) | Inhibition of aminopeptidases by amastatin and bestatin derivatives. Effect of inhibitor structure on slow-binding processes: D.H. Rich, et al.; J. Med. Chem. 27, 417 (1984) | The slow, tight binding of bestatin and amastatin to aminopeptidases: S.H. Wilkes & J.M. Prescott; J. Biol. Chem. 260, 13154 (1985) | Inhibition of aminopeptidases by peptides containing ketomethylene and hydroxyethylene amide bond replacements: S.L. Harbeson & D.H. Rich; J. Med. Chem. 32, 1378 (1989) | Role of aminopeptidase activity in the regulation of the pressor activity of circulating angiotensins: S. Ahmad & P.E. Ward; J. Pharmacol. Exp. Ther. 252, 643 (1990) | Purification and biochemical characterization of recombinant simian immunodeficiency virus protease and comparison to human immunodeficiency virus type 1 protease: S.K. Grant, et al.; Biochemistry 30, 8424 (1991) | Rat liver mitochondrial intermediate peptidase (MIP): purification and initial characterization: F. Kalousek, et al.; EMBO J. 11, 2803 (1992) | Vasopressin and amastatin induce V(1)-receptor-mediated suppression of excitatory transmission in the rat parabrachial nucleus: X. Chen & Q.J. Pittman; J. Neurophysiol. 82, 1689 (1999) | Inhibitors on an elastase-like enzyme activity catalyzing Suc-Ala-Ala-Pro-Leu-pNA amidolysis in human seminal plasma: Y. Matsuda, et al.; Arch. Androl. 44, 1 (2000) | The most potent organophosphorus inhibitors of leucine aminopeptidase. Structure-based design, chemistry, and activity: J. Grembecka, et al.; J. Med. Chem. 46, 2641 (2003) | Glutamine-181 is crucial in the enzymatic activity and substrate specificity of human endoplasmic-reticulum aminopeptidase-1: Y. Goto, et al.; Biochem. J. 416, 109 (2008) | Angiotensin III modulates the nociceptive control mediated by the periaqueductal gray matter: A. Pelegrini-da-Silva, et al.; Neurosci. 164, 1263 (2009) | Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses: F. Qi, et al.; BBRC (Epub ahead of print) (2020)
Related Products
| Product Name | Product Code | Supplier | AEBSF . hydrochloride | AG-CR1-3610 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|