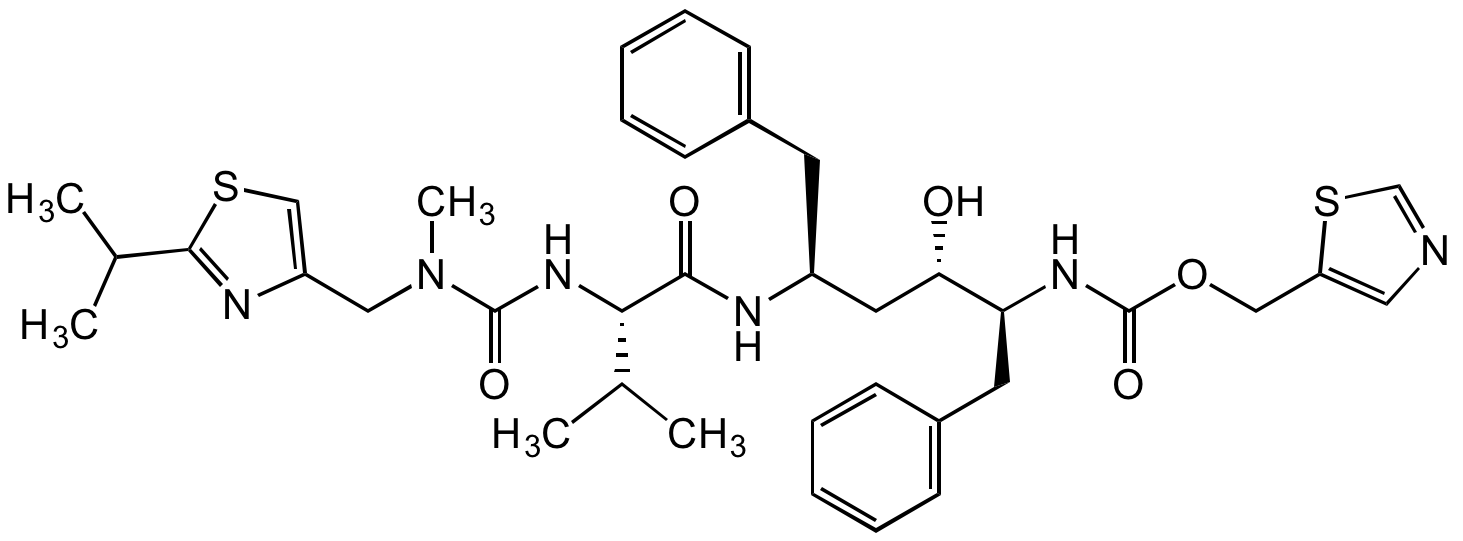
Ritonavir
| Code | Size | Price |
|---|
| AG-CR1-3683-M010 | 10 mg | £45.00 |
Quantity:
| AG-CR1-3683-M050 | 50 mg | £110.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Target Species: Universal
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
RTV; A-84538; ABT-538; Abbot 84538; NSC 693184
Appearance:
White to off-white solid.
CAS:
155213-67-5
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302
InChi:
InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1
InChiKey:
NCDNCNXCDXHOMX-XGKFQTDJSA-N
Long Description:
Chemical. CAS: 155213-67-5. Formula: C37H48N6O5S2. MW: 720.9. . HIV-1 and HIV-2 protease inhibitor. Glucose transporter 1 (GLUT1) and 4 (GLUT4) inhibitor. Inhibits transport of glucose across the plasma membranes of mammalian cells and consequently decreases glycolysis. Useful agent for immunometabolism research. Antitumor agent. Apoptosis inducer and cell proliferation inhibitor in GLUT1/GLUT4 overexpressed cancer cell lines. Tumor cells rely on elevated glucose consumption and metabolism for survival and proliferation. Glucose transporters mediating glucose entry are key proximal rate-limiting checkpoints. Among the fourteen SLC2A family members, GLUTs 1 and 4 are high-affinity glucose transporters.
MDL:
MFCD00927142
Molecular Formula:
C37H48N6O5S2
Molecular Weight:
720.9
Package Type:
Vial
Precautions:
P301+P312
Product Description:
HIV-1 and HIV-2 protease inhibitor. Glucose transporter 1 (GLUT1) and 4 (GLUT4) inhibitor. Inhibits transport of glucose across the plasma membranes of mammalian cells and consequently decreases glycolysis. Useful agent for immunometabolism research. Antitumor agent. Apoptosis inducer and cell proliferation inhibitor in GLUT1/GLUT4 overexpressed cancer cell lines. Tumor cells rely on elevated glucose consumption and metabolism for survival and proliferation. Glucose transporters mediating glucose entry are key proximal rate-limiting checkpoints. Among the fourteen SLC2A family members, GLUTs 1 and 4 are high-affinity glucose transporters. Inhibits 20S proteasome chymotrypsin-like activity. The proteinase inhibitor Kaletra, which is a mixture of the HIV-1 proteinase inhibitors lopinavir and ritonavir has been shown to be effective against SARS-CoV and MERS-CoV. Shown in a SARS-CoV-2 protease structure model study to potentially bind and inhibit the coronavirus endopeptidase C30 (CEP_C30) of SARS-CoV-2. An initial randomized trial study was not successful.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
CC(C1=NC(CN(C(N[C@H](C(N[C@H](C[C@@H]([C@@H](NC(OCC2=CN=CS2)=O)CC3=CC=CC=C3)O)CC4=CC=CC=C4)=O)C(C)C)=O)C)=CS1)C
Solubility Chemicals:
Soluble in DMSO (10mg/ml), DMF (10mg/ml) or EtOH.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans: D.J. Kempf, et al.; PNAS 92, 2484 (1995) | Discovery of ritonavir, a potent inhibitor of HIV protease with high oral bioavailability and clinical efficacy: D.J. Kempf, et al.; J. Med. Chem. 41, 602 (1998) | How an Inhibitor of the HIV-I Protease Modulates Proteasome Activity: G. Schmidtke, et al.; J. Biol. Chem. 274, 35734 (1999) | Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients: R.K. Zeldin, et al.; J. Antimicrob. Chemother. 53, 4 (2004) | Molecular dynamic simulations analysis of ritonavir and lopinavir as SARS-CoV 3CL(pro) inhibitors: V. Nukoolkarn, et al.; J. Theor. Biol. 254, 861 (2008) | HIV protease inhibitors act as competitive inhibitors of the cytoplasmic glucose binding site of GLUTs with differing affinities for GLUT1 and GLUT4: R.C. Hresko & P.W. Hruz; PLoS One 6, e25237 (2011) | Targeting Glycolysis and Compensatory Mitochondrial Metabolism In An In Vivo Xenograft Model Of Multiple Myeloma With FDA Approved Ritonavir and Metformin: S. Dalva-Aydemir, et al.; Blood 122, 1922 (2013) | Glucose transporter 1-mediated glucose uptake is limiting for B-cell acute lymphoblastic leukemia anabolic metabolism and resistance to apoptosis: T. Liu, et al.; Cell Death Dis. 5, e1470 (2014) | In Silico Modeling-based Identification of Glucose Transporter 4 (GLUT4)-selective Inhibitors for Cancer Therapy: R.K. Mishra, et al.; J. Biol. Chem. 290, 14441 (2015) | A guide to immunometabolism for immunologists: L.A. O'Neill, et al.; Nat. Rev. Immunol. 16, 553 (2016) | Development of GLUT4-selective antagonists for multiple myeloma therapy: C. Wei, et al.; Eur. J. Med. Chem. 139, 573 (2017) | Molecular Modeling Evaluation of the Binding Effect of Ritonavir, Lopinavir and Darunavir to Severe Acute Respiratory Syndrome Coronavirus 2 Proteases: S. Lin, et al.; (Epub ahead of print) (2020) | A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19: B. Cao, et al.; N. Engl. J. Med. (Epub ahead of print) (2020)
Related Products
| Product Name | Product Code | Supplier | 2-Deoxy-D-glucose | AG-CR1-3681 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|