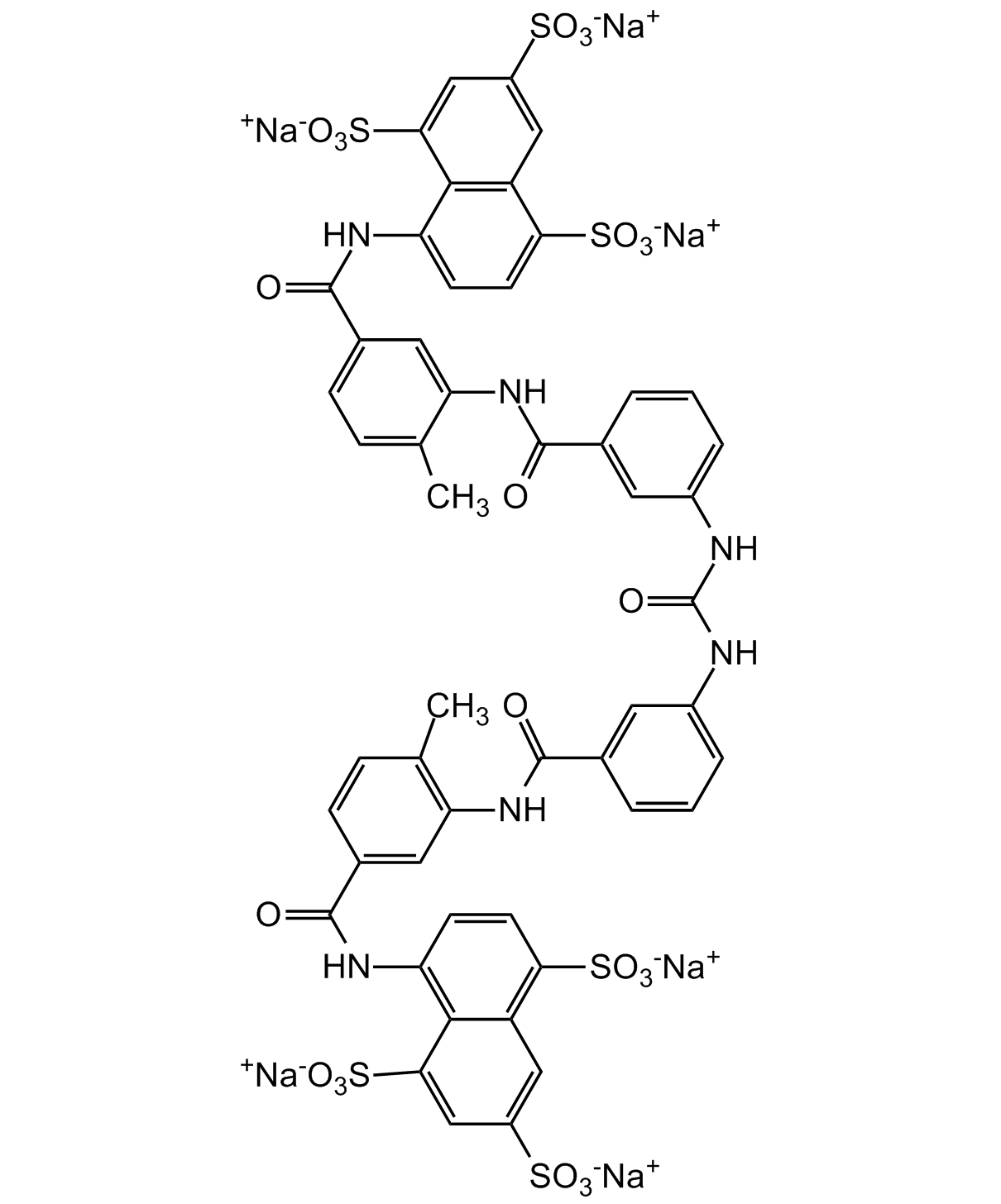
Suramin . hexasodium salt
| Code | Size | Price |
|---|
| AG-CR1-3575-M050 | 50 mg | £80.00 |
Quantity:
| AG-CR1-3575-M250 | 250 mg | £290.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short term storage:+4°C. Long term storage:+4°C.
Images
Documents
Further Information
Alternate Names/Synonyms:
Germanin; NSC 34936; SK 24728
Appearance:
White to off-white powder.
CAS:
129-46-4
EClass:
32160000
Form (Short):
solid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C51H40N6O23S6.6Na/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80;;;;;;/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80);;;;;;/q;6*+1/p-6
InChiKey:
VAPNKLKDKUDFHK-UHFFFAOYSA-H
Long Description:
Chemical. CAS: 129-46-4. Formula: C51H34N6O23S6 . 6Na. MW: 1291.2 . 137.9. Synthetic. Potent ATPase inhibitor. Potent competitive inhibitor of reverse transcriptase. Shows anti-HIV activity. Anticancer compound. Protein kinase C (PKC) inhibitor. Potent inhibitor of melanoma heparanase and tumor cell metastasis. Non-specific growth factors inhibitor (including PDGF, EGF, aFGF and bFGF). TGF-beta1 inhibitor. Topoisomerase I and II inhibitor. Interleukin-1 (IL-1) inhibitor. Interleukin-4 (IL-4) inhibitor. G protein inhibitor. P2X and P2Y purinergic receptor antagonist. Antiangiogenic. Potent VEGF inhibitor. Telomerase inhibitor. Shows adjuvant properties. Regulates ryanodine receptor. Direct adenylyl cyclase inhibitor. Protein synthesis inhibitor. SIRT1 (sirtuin 1) and SIRT5 (sirtuin 5) inhibitor. Immunosuppressive. Antifibrotic agent. Antiparasitic. Antiprotozoal. Athelmintic. Cullin-RING E3 ubiquitin ligase inhibitor.
MDL:
MFCD00210217
Molecular Formula:
C51H34N6O23S6 . 6Na
Molecular Weight:
1291.2 . 137.9
Package Type:
Vial
Product Description:
Potent ATPase inhibitor [1]. Potent competitive inhibitor of reverse transcriptase. Shows anti-HIV activity [2, 3]. Anticancer compound [4, 5, 15]. Protein kinase C (PKC) inhibitor [4]. Potent inhibitor of melanoma heparanase and tumor cell metastasis [6]. Non-specific growth factors inhibitor (including PDGF, EGF, aFGF and bFGF) [7, 16]. TGF-beta1 inhibitor [8]. Topoisomerase I and II inhibitor [9]. Interleukin-1 (IL-1) inhibitor [10]. Interleukin-4 (IL-4) inhibitor [11]. G protein inhibitor [12]. P2X and P2Y purinergic receptor antagonist [13]. Antiangiogenic. Potent VEGF inhibitor [14, 15]. Telomerase inhibitor [17]. Shows adjuvant properties [18]. Regulates ryanodine receptor [19]. Direct adenylyl cyclase inhibitor [20]. Protein synthesis inhibitor [21]. SIRT1 (sirtuin 1) and SIRT5 (sirtuin 5) inhibitor [22, 23]. Immunosuppressive [24]. Antifibrotic agent [25]. Antiparasitic. Antiprotozoal. Athelmintic [26]. Cullin-RING E3 ubiquitin ligase inhibitor [27]. Inhibitor of the STING pathway via the inhibition of cGAMP synthase (cGAS) enzymatic activity. Inhibits SARS-CoV-2 infection in cell culture by blocking early steps (binding/fusion) of the replication cycle. Potentially binds and inhibits nsp12 of SARS-CoV-2, binding to motifs harbouring the RNA-dependent RNA polymerases (RdRps) activity.
Purity:
>98% (Pharmaceutical Grade).
SMILES:
[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].CC1=CC=C(C=C1NC(=O)C1=CC(NC(=O)NC2=CC=CC(=C2)C(=O)NC2=C(C)C=CC(=C2)C(=O)NC2=C3C(C=C(C=C3S([O-])(=O)=O)S([O-])(=O)=O)=C(C=C2)S([O-])(=O)=O)=CC=C1)C(=O)NC1=CC=C(C2=C1C(=CC(=C2)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O
Solubility Chemicals:
Soluble in water or DMSO. Sparingly soluble in ethanol.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
Suramin: a potent ATPase inhibitor which acts on the inside surface of the sodium pump: P.A. Fortes, et al.; Biochim. Biophys. Acta 318, 262 (1973) | Suramin: a potent inhibitor of the reverse transcriptase of RNA tumor viruses: E. De Clerq; Cancer Lett. 8, 9 (1979) | Suramin protection of T cells in vitro against infectivity and cytopathic effect of HTLV-III: H. Mitsuya, et al.; Science 226, 172 (1984) | Suramin, an anti-cancer drug, inhibits protein kinase C and induces differentiation in neuroblastoma cell clone NB2A: C.E. Hensey, et al.; FEBS Lett. 258, 156 (1989) | Suramin: prototype of a new generation of antitumor compounds: R.V. La Rocca, et al.; Cancer Cells 2, 106 (1990) (Review) | Suramin. A potent inhibitor of melanoma heparanase and invasion: M. Nakajima, et al.; J. Biol. Chem. 266, 9661 (1991) | Nature of the interaction of growth factors with suramin: C.R. Middaugh, et al.; Biochemistry 31, 9016 (1992) | The antiproliferative effect of suramin on the cancer cell line SW-13 is mediated by the inhibition of transforming growth factor beta 1 (TGF-beta 1): R. Danesi, et al.; Pharmacol. Res. 25, 17 (1992) | Suramin inhibits DNA damage in human prostate cancer cells treated with topoisomerase inhibitors in vitro: H. Yamazaki, et al.; Prostate 23, 25 (1993) | Suramin blocks the binding of interleukin-1 to its receptor and neutralizes IL-1 biological activities: G. Strassmann, et al.; Int. J. Immunopharmacol. 16, 931 (1994) | Suramin blocks binding of interleukin-4 to its receptors on human tumor cells and interleukin-4-induced mitogenic response: P. Leland, et al.; Oncol. Res. 7, 227 (1995) | Suramin analogues as subtype-selective G protein inhibitors: M. Freissmuth, et al.;~Mol. Pharmacol. 49, 602 (1996) | PPADS and suramin as antagonists at cloned P2Y- and P2U-purinoceptors: S.J. Charlton, et al.; Br. J. Pharmacol. 118, 704 (1996) | Suramin is a potent inhibitor of vascular endothelial growth factor. A contribution to the molecular basis of its antiangiogenic action: J. Waltenberger, et al.; J. Mol. Cell Cardiol. 28, 1523 (1996) | Antiangiogenic and antiproliferative activity of suramin analogues: A.R. Gagliardi, et al.; Cancer Chemother. Pharmacol. 41, 117 (1998) | Suppression of myocardial inflammation using suramin, a growth factor blocker: T. Shiono, et al.;~Circ. J. 66, 385 (2002) | Suramin suppresses growth, alkaline-phosphatase and telomerase activity of human osteosarcoma cells in vitro: K. Trieb & H. Blahovec; Int. J. Biochem. Cell Biol. 35, 1066 (2003) | Suramin has adjuvant properties and promotes expansion of antigen-specific Th1 and Th2 cells in vivo: M. Denkinger, et al.; Int. Immunopharmacol. 4, 15 (2004) | Functional regulation of the cardiac ryanodine receptor by suramin and calmodulin involves multiple binding sites: A.P. Hill, et al; Mol. Pharmacol. 65, 1258 (2004) | Modulation of adenylyl cyclase activity in young and adult rat brain cortex. Identification of suramin as a direct inhibitor of adenylyl cyclase: J. St?hr, et al.; J. Cell Mol. Med. 9, 940 (2005) | Inhibition by suramin of protein synthesis in vitro. Ribosomes as the target of the drug: M. Brigotti, et al.;~Biochimie 88, 497 (2006) | Structure-activity studies on suramin analogues as inhibitors of NAD+-dependent histone deacetylases (sirtuins): J. Trapp, et al.; ChemMedChem 2, 1419 (2007) | Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin: A. Schuetz, et al.; Structure 15, 377 (2007) | Suramin inhibits the CD40-CD154 costimulatory interaction: a possible mechanism for immunosuppressive effects: E. Margolles-Clark, et al.; Biochem. Pharmacol. 77, 1236 (2009) | Tissue protective and anti-fibrotic actions of suramin: new uses of an old drug: N. Liu & S. Zhuang; Curr. Clin. Pharmacol. 6, 137 (2011) (Review) | The trypanocidal drug suramin and other trypan blue mimetics are inhibitors of pyruvate kinases and bind to the adenosine site: H.P. Morgan, et al.; J. Biol. Chem. 286, 31232 (2011) | Suramin inhibits cullin-RING E3 ubiquitin ligases: K. Wu, et al.; PNAS 113, E2011 (2016) | Suramin potently inhibits cGAMP synthase, cGAS, in THP1 cells to modulate IFN-beta levels: M. Wang, et al.; Future Med. Chem. 10, 1301 (2018) | Suramin inhibits SARS-CoV-2 infection in cell culture by interfering 2 with early steps of the replication cycle: C. Salgado, et al.; Antimicrob. Agents Chemother. 64, e00900 (2020) | Suramin, Penciclovir and Anidulafungin bind nsp12, which governs the RNA-dependent-RNA polymerase activity of SARS-CoV-2, with higher interaction energy than Remdesivir, indicating potential in the treatment of Covid-19 infection: S.K. Dey, et al.; (OSF Preprints) (2020)