| | Type | Category | Supplier | |
|---|
| |
 | General processes for regulating ferroptosis are associated with iron, lipid, and amino acid metabolism. |
Documents | Cell Cycle | Abnova Corporation | |
 | Abnova provides a range of antibodies specifically for melanomas. |
Documents | Cancer | Abnova Corporation | |
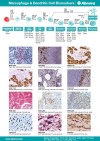 | Abnova provides a range of Macrophage & Dendritic Cell Biomarkers. |
Documents | Immunology | Abnova Corporation | |
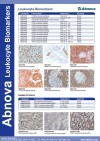 | Abnova provides a range of leukocyte biomarker antibodies. |
Documents | Antibodies | Abnova Corporation | |
 | HIFs induce the expression of integrins. The integrins connect the ECM to the cytoskeleton and form focal adhesion, which enhances the cancer cell motility and invasion capacity. HIFs can also trigger collagen production and regulate the expression of collagen-modifying enzymes. The hypoxia-induced collagen deposition might potentiate cancer metastasis. |
Documents | Cancer | Abnova Corporation | |
 | Infectious disease rapid tests enable the simultaneous detection of multiple markers in serum, plasma, cerebrospinal, and synovial fluid. They are an important tool for the differential study of diseases with similar symptoms. They are used for the confirmation of borderline or positive ELISA. |
Documents | Immunology | Abnova Corporation | |
 | Abnova Corporation provides a range of Immune Checkpoint Receptors. |
Documents | Immunology | Abnova Corporation | |
 | Abnova provides a range of Histone antibodies and kits. |
Documents | Antibodies | Abnova Corporation | |
 | Abnova provides a range of FISH probes for clinical applications. |
Documents | Probes | Abnova Corporation | |
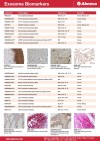 | Abnova provides a range of exosome biomarker antibodies. |
Documents | Antibodies | Abnova Corporation | |
 | Abnova provide a range of antibodies specifically for endometrial cancer. |
Documents | Cancer | Abnova Corporation | |
 | miRNA probes are short RNA molecules used to identify and localize microRNAs (miRNAs) present in biological samples. They are designed to be complementary to the target miRNA sequence, allowing them to form a stable duplex with the miRNA and facilitating the detection and quantification of miRNAs. With Abnovas state-of-the-art custom miRNA probe service, they offer you the power to design and tailor high-quality miRNA probes specific to your research needs. |
Documents | Service | Abnova Corporation | |
 | Circulating tumour cell (CTC)-derived xenografts (CDx) offer an opportunity for those patients that cannot undergo an invasive procedure to generate tumoUr models and assess their potential roles in tumour progression and management. |
Documents | Cancer | Abnova Corporation | |
 | Abnova provides a range of antibodies specifically for colorectal cancer. |
Documents | Cancer | Abnova Corporation | |
 | Abnova provide a range of Cancer Epigenetics polyclonal and monoclonal antibodies |
Documents | Cancer | Abnova Corporation | |
 | Abnova provide a range of recombinant monoclonal antibody cancer biomarkers. |
Documents | Cancer | Abnova Corporation | |
 | Product list of Bioreagents for FDA-Approved Therapeutic Mabs and Anti-Drug Mabs |
Documents | ELISA | Abnova Corporation | |
 | Abnova Bioactive Proteins Product Lists |
Documents | Proteins | Abnova Corporation | |
 | A selection of Abnovas Best-in-Class Bioreagents |
Documents | Catalogue | Abnova Corporation | |
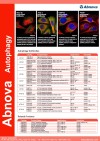 | Abnova provides a range of Autophagy antibodies. |
Documents | Autophagy | Abnova Corporation | |
 | HER2, EGFR, HER3, and IGF-1R dimerized with the HER2 receptor induce activation of PI3K/AKT and MAPK/MEK signalling pathways and receive signals to regulate the downstream cascade that promotes the active transcription of genes involved in proliferation, angiogenesis, cell survival, and metastasis. |
Documents | Transcription Factors | Abnova Corporation | |
 | Abnova provides a range of Apoptosis biomarkers. |
Documents | Apoptosis | Abnova Corporation | |
 | Abnova Corporation provides Antibodies & Proteins Specific for Coronaviruses |
Documents | COVID-19 | Abnova Corporation | |
 | Abnova Corporation provides a range of Acute Myeloid Leukemia Antibodies. |
Documents | Cancer | Abnova Corporation | |
 | Abnova offers Antibody Drug ELISA Kits. |
Documents | ELISA | Abnova Corporation | |