AdipoGen Life Sciences specialises in the development of recombinant proteins that show enhanced activity and stability, produced in mammalian cells. Using proprietary in-house technologies together with established and published technologies, they engineer, develop and produce an innovative panel of recombinant proteins for different research fields including Cell Therapy Research.
The development of recombinant proteins is performed using different production expression systems, including the mammalian cell lines CHO and HEK 293. The mammalian cell lines allow AdipoGen Life Sciences to produce exogenous recombinant proteins secreted into the cell medium with post-translational modifications and glycosylations closer to endogenous proteins compared to productions in E. coli. However, for some difficult-to-produce in mammalian cells proteins, they also use bacteria (E. coli) and/or insect cells as a host. AdipoGen Life Sciences’ first goal is to provide high purity, low endotoxin and biologically active recombinant proteins, leveraging its in-house protein technologies.
Recombinant proteins are used throughout the life science academia and industry in a plethora of applications. They elicit their mechanism of action by activating signalling pathways through binding to specific cell-surface receptors. Their use ranges from cell culture to bioprocessing and advanced cell and gene therapy. A defined composition of the cell culture medium to guarantee batch-to-batch reproducibility has become inevitable. The development of animal-component-free recombinant proteins eliminates the risk of contamination that has been associated with animal and human serum-derived media.
AdipoGen Life Sciences’ initiative to implement the production in serum-free (animal-component free) media conditions, copes with the need for its highly biologically active and stable proteins for these newly emerging markets and helps advance life sciences research.
TNF Ligands Multimeric Proteins
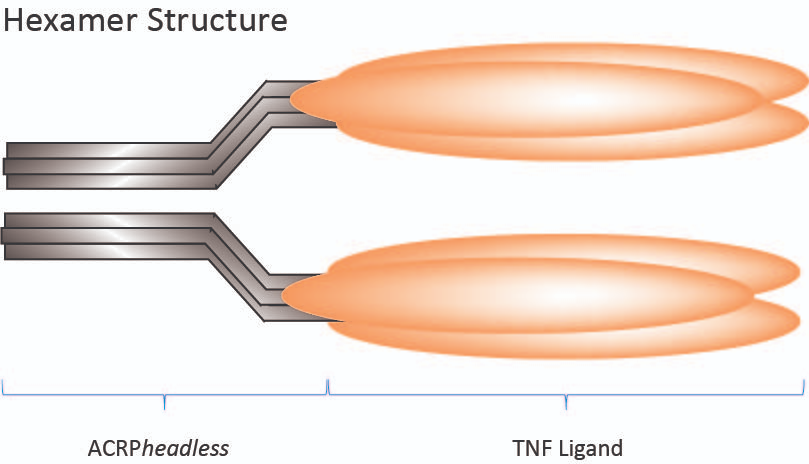
- Endogenous TNF superfamily ligands are either active as membrane-form (e.g. FasL, TRAIL, CD40L, OX40L) or are secreted and activated through oligomerisation by the binding of proteoglycans at the surface of cells (e.g. APRIL).
- MultimericLigands™ are constructs in which two trimeric TNFSF ligands are linked via the oligomeric collagen domain of Adiponectin [ACRP30headless].
- MultimericLigands™ mimic the membrane-bound forms of the proteins and show high activity.
- For in vitro studies!
COMP-Fusion Proteins – Improved Avidity & Biological Activity
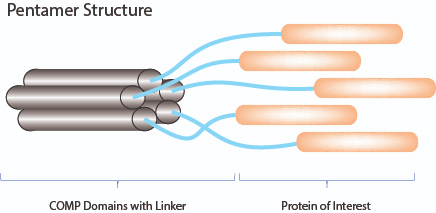
- The avidity (binding) and activity of selected proteins are improved by the addition of an oligomerisation domain fused at the N- or C-terminus of the protein of interest.
- COMP-Fusion Proteins are based on the pentamerisation domain (minimal coiled-coil domain) of the cartilage oligomeric matrix protein (COMP) which is fused through a specific linker to proteins of interest.
- Using this technology AdipoGen Life Sciences generates cytokines with improved avidity and biological activity.
- For in vitro and in vivo studies!
Monomeric Proteins fused to Fc (KIH-Technology)
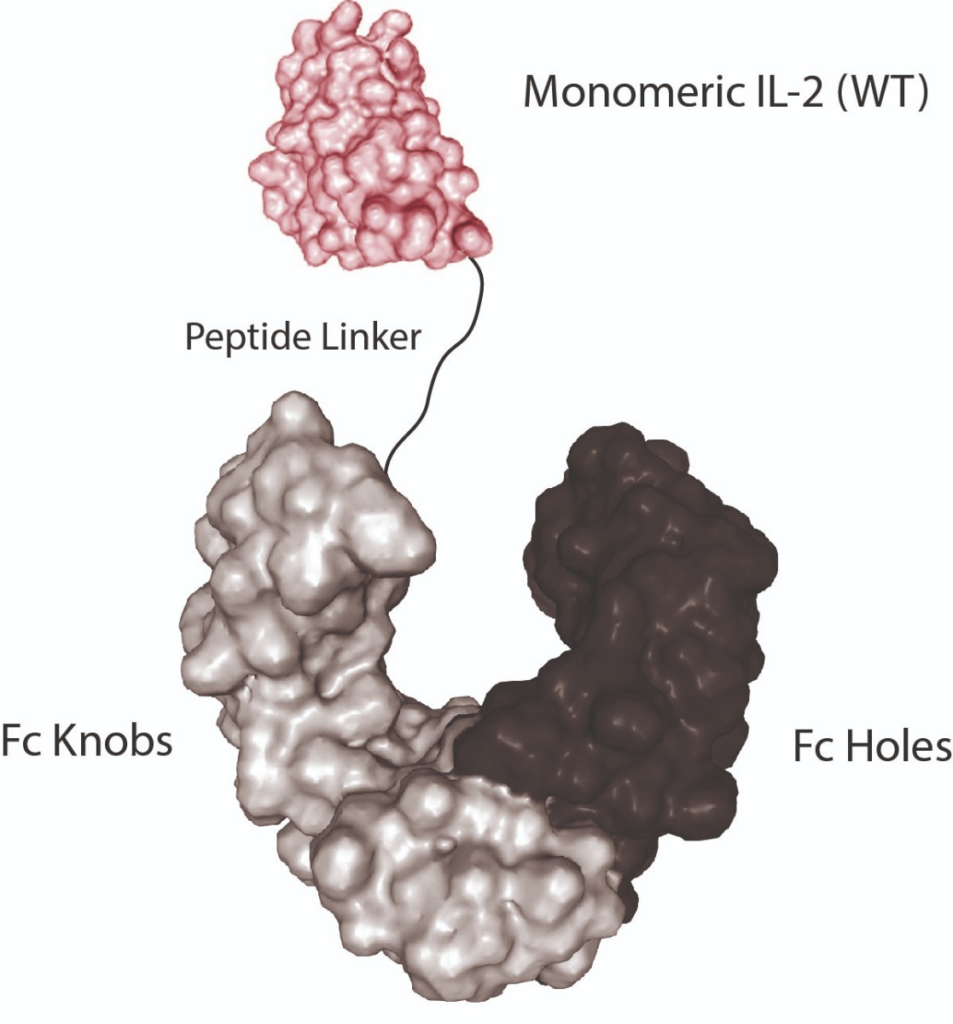
- The knobs-into-holes (KIH) strategy is a pioneering format, permitting heavy-chain heterodimerisation to create an “IgG with two different arms”.
- The KIH technology can also be used for protein engineering.
- AdipoGen Life Sciences developed various interleukins (e.g. IL-2, IL-33 and IL-38) into an Fc-fusion protein using the KIH technology.
- The KIH proteins are monomeric (see Figure) with greatly enhanced stability and substantially improved pharmacokinetics (PK) while maintaining cytokine activity.
- AdipoGen Life Sciences also developed heterodimeric proteins using the KIH-technology.
- These special monomeric Fc-constructs are produced in mammalian cells with low endotoxin content.
- For in vitro and in vivo studies!
Knobs-into-Holes (KIH) Technology:
Lit: J.B. Ridgway, et al.; Protein Eng. 9, 617 (1996)
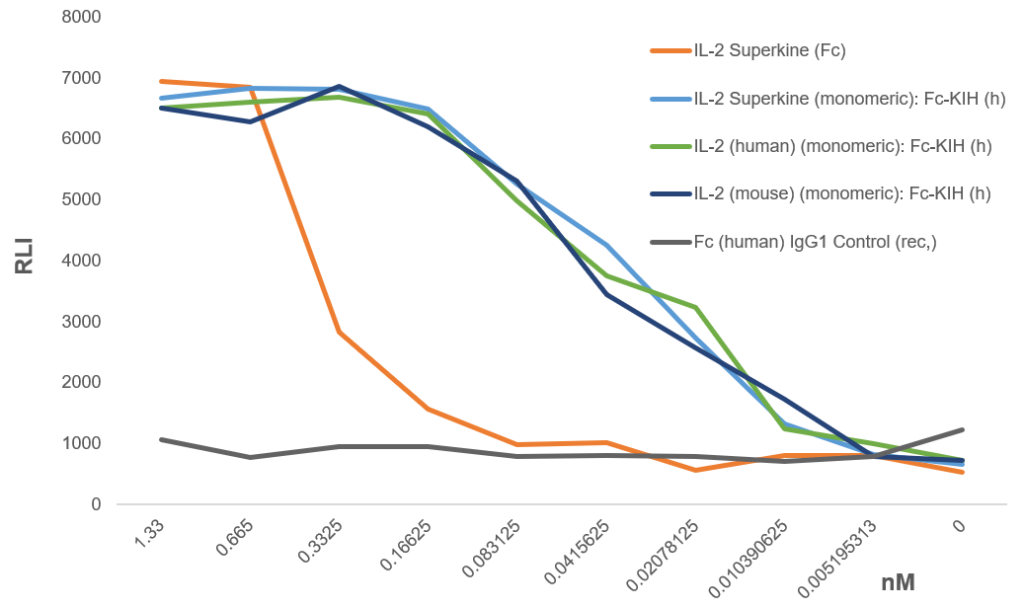
The monomeric IL-2 proteins, including the IL-2 (human) (monomeric):Fc-KIH (human) (rec.) (AG-40B-0224), IL-2 (mouse) (monomeric):Fc-KIH (human) (rec.) (AG-40B-0225) and IL-2 Superkine (monomeric):Fc-KIH (human) (rec.) (AG-40B-0222) are activity tested and compared to a dimeric Fc fusion protein IL-2 Superkine (Fc) (AG-40B-0111), all produced in mammalian cells with low endotoxin content. Activation of IL-2R is analysed using the IL-2 Bioassay from Promega (JA2201) consisting of a genetically engineered cell line expressing the IL-2R. When IL-2 binds to its receptor (IL-2R), receptor-mediated pathway signalling induces luminescence that is measured with a luminometer. The experiment shows a several-fold increased activity of the monomeric constructs.
Fc-Fusion Proteins (wild-type or non-lytic)
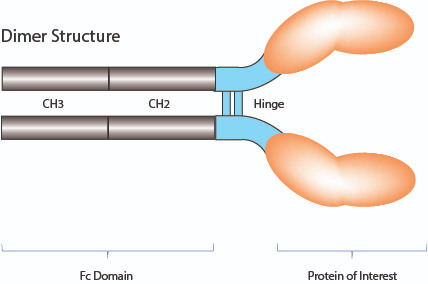
- Fc-Fusion proteins (also known as Fc-Chimeric Fusion proteins, Ig-based Chimeric Fusion proteins and Fc-tag proteins) are composed of the Fc domain of IgG genetically linked to a protein of interest. Dimeric structures are formed during the production of mammalian cells.
- The fusion of a cytokine sequence to the Fc domain of an IgG (human or mouse) determines a prolonged circulating half-life time in vivo.
- The Fc domain folds independently and can improve the solubility and stability of the partner molecule both in vitro and in vivo.
- Non-lytic: Mutations to the complement (C1q) and FcgR I binding sites of the IgGs Fc fragment render the fusion proteins incapable of antibody-directed cytotoxicity (ADCC) and complement-directed cytotoxicity (CDC).
- For in vitro and especially in vivo studies!
Proteins with Site-directed Mutations
Site-directed mutagenesis is a valuable tool to modify genes and study the structural and functional properties of a protein, based on the structure, function, catalytic mechanism and catalytic residues of enzymes and therefore of high importance for protein engineering. The mutation may be a single base change (point mutation), multiple base changes (many mutations), deletion or insertion.
Selected Other Specialty Proteins
AdipoGen Life Sciences’ R&D department has established in-house protocols for certain speciality proteins. These proteins have a special structure, multimerisation or no tags. Only due to these special production protocols do these proteins elicit their biological activity. See here for a complete list of Untagged Proteins.
Originally posted on adipogen.com/enhanced-proteins
Caltag Medsystems is the distributor of Adipogen products in the UK and Ireland. If you have any questions about these products, please contact us.
