SARS-CoV-2 Neutralizing Antibodies Detection Kit
Product Code:
AG-48B-0002
AG-48B-0002
Regulatory Status:
RUO
RUO
Shipping:
Blue Ice
Blue Ice
Storage:
Short term: +4°C. Long term: +4°C
Short term: +4°C. Long term: +4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-48B-0002-KI01 | 96 wells | £691.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges to UK mainland customers, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
COVID-19 Neutralizing Antibodies Detection Kit; 2019-nCoV Neutralizing Antibodies Detection Kit; 2019-nCoV Spike Protein S1 (RBD) Neutralizing Antibodies Detection Kit
Detection Type:
Colorimetric
EClass:
32160000
Handling Advice:
After standard reconstitution, prepare aliquots and store at -20°C.Avoid freeze/thaw cycles.Plate and reagents should reach room temperature before use.
Long Description:
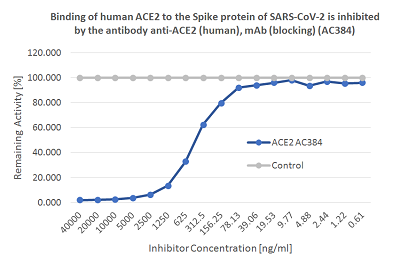
Screening Assay: To measure the presence of neutralizing/blocking antibodies in human serum/plasma that inhibit the binding of the SARS-CoV-2 Spike (RBD) protein to its human receptor ACE2. Coronaviruses (CoVs) are enveloped non-segmented positive-sense single-stranded RNA viruses and can infect respiratory, gastrointestinal, hepatic and central nervous system of human and many other wild animals. Recently, a new severe acute respiratory syndrome beta-coronavirus called SARS-CoV-2 has emerged, which causes an epidemic of acute respiratory syndrome (called coronavirus human disease 2019 or COVID-19). SARS-CoV-2 contains 4 structural proteins, including Envelope (E), Membrane (M), Nucleocapsid (N) and Spike (S), which is a transmembrane protein, composed of two subunits S1 and S2. The S1 subunit contains a receptor binding domain (RBD), which binds to the cell surface receptor Angiotensin-Converting Enzyme 2 (ACE2) present at the surface of epithelial cells, causing mainly infection of human respiratory cells.The SARS-CoV-2 Neutralizing Antibodies Detection Kit contains key reagents required to test the presence of functional neutralizing antibodies against SARS-CoV-2 present in the serum or plasma. It is an easy and fast alternative to the classical neutralization assay using Vero E6 cells. This Detection Kit is based on a colorimetric reaction, which measures the binding of the RBD of the Spike S protein from SARS-CoV-2 to its human receptor ACE2. The presence of neutralizing / blocking antibodies in the samples are detected by reduction of signal indicating the inhibition of the Spike-ACE2 binding.
Package Type:
Plastic Vial
Product Description:
Coronaviruses (CoVs) are enveloped non-segmented positive-sense single-stranded RNA viruses and can infect respiratory, gastrointestinal, hepatic and central nervous system of human and many other wild animals. Recently, a new severe acute respiratory syndrome beta-coronavirus called SARS-CoV-2 has emerged, which causes an epidemic of acute respiratory syndrome (called coronavirus human disease 2019 or COVID-19). SARS-CoV-2 contains 4 structural proteins, including Envelope (E), Membrane (M), Nucleocapsid (N) and Spike (S), which is a transmembrane protein, composed of two subunits S1 and S2. The S1 subunit contains a receptor binding domain (RBD), which binds to the cell surface receptor Angiotensin-Converting Enzyme 2 (ACE2) present at the surface of epithelial cells, causing mainly infection of human respiratory cells.The SARS-CoV-2 Neutralizing Antibodies Detection Kit contains key reagents required to test the presence of functional neutralizing antibodies against SARS-CoV-2 present in the serum or plasma. It is an easy and fast alternative to the classical neutralization assay using Vero E6 cells. This Detection Kit is based on a colorimetric reaction, which measures the binding of the RBD of the Spike S protein from SARS-CoV-2 to its human receptor ACE2. The presence of neutralizing / blocking antibodies in the samples are detected by reduction of signal indicating the inhibition of the Spike-ACE2 binding.
Sample Type:
Plasma, Serum
Specificity:
To measure the presence of neutralizing/blocking antibodies in human serum/plasma that inhibit the binding of the SARS-CoV-2 Spike (RBD) protein to its human receptor ACE2.
Transportation:
Non-hazardous
UNSPSC Category:
General Purpose Cell Biology Kits
UNSPSC Number:
41116111
Use & Stability:
12 months after the day of manufacturing. See expiry date on ELISA Kit box.
References
Comparison of two commercial surrogate ELISAs to detect a neutralizing antibody response to SARS-CoV-2: K. Mueller, et al.; J. Virol. Methods 292, 114122 (2021) https://www.sciencedirect.com/science/article/pii/S0166093421000616 | Unique autoantibody prevalence in long-term recovered SARS-CoV-2-infected individuals: H. Lingel, et al.; J. Autoimmun. 122, 102682 (2021)